Cyclic maltosyl maltose, cyclic maltosyl maltose synthase, method of producing the same and use thereof
A maltose and cyclic technology, applied in the field of cyclic maltosyl maltose and cyclic maltosyl maltose synthetase and their production and application, can solve the problems of strong reactivity, small molecules, browning, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0302] Arthrobacter globosa M6 (FERM BP-8448) was seed cultured according to the method in Experiment 3. Then, about 20 L of partially decomposed starch (trade name Pindex #100, manufactured by Matsutani Chemical Industry Co., Ltd.) 3.0 w / v% and soybean peptide (trade name Hiniut SMS, produced by Fuji) were added to a fermenter with a capacity of 30 L. Oil Co., Ltd.) 3.6w / v%, dipotassium hydrogenphosphate 0.1w / v%, sodium dihydrogenphosphate dihydrate 0.06w / v%, magnesium sulfate heptahydrate 0.05w / v%, calcium carbonate 0.3w / The liquid culture medium composed of v% and water was heat-sterilized, cooled, and after reaching a temperature of 27°C, inoculated with 1v / v% of the seed culture solution, maintained at a temperature of 27°C, pH 5.5 to 8.0, and carried out aerated culture for 96 hours. After the cultivation, use SF membrane to perform sterilizing filtration, recover about 18 L of culture filtrate, then concentrate the filtrate with UF membrane, and recover about 1 L of co...
Embodiment 2
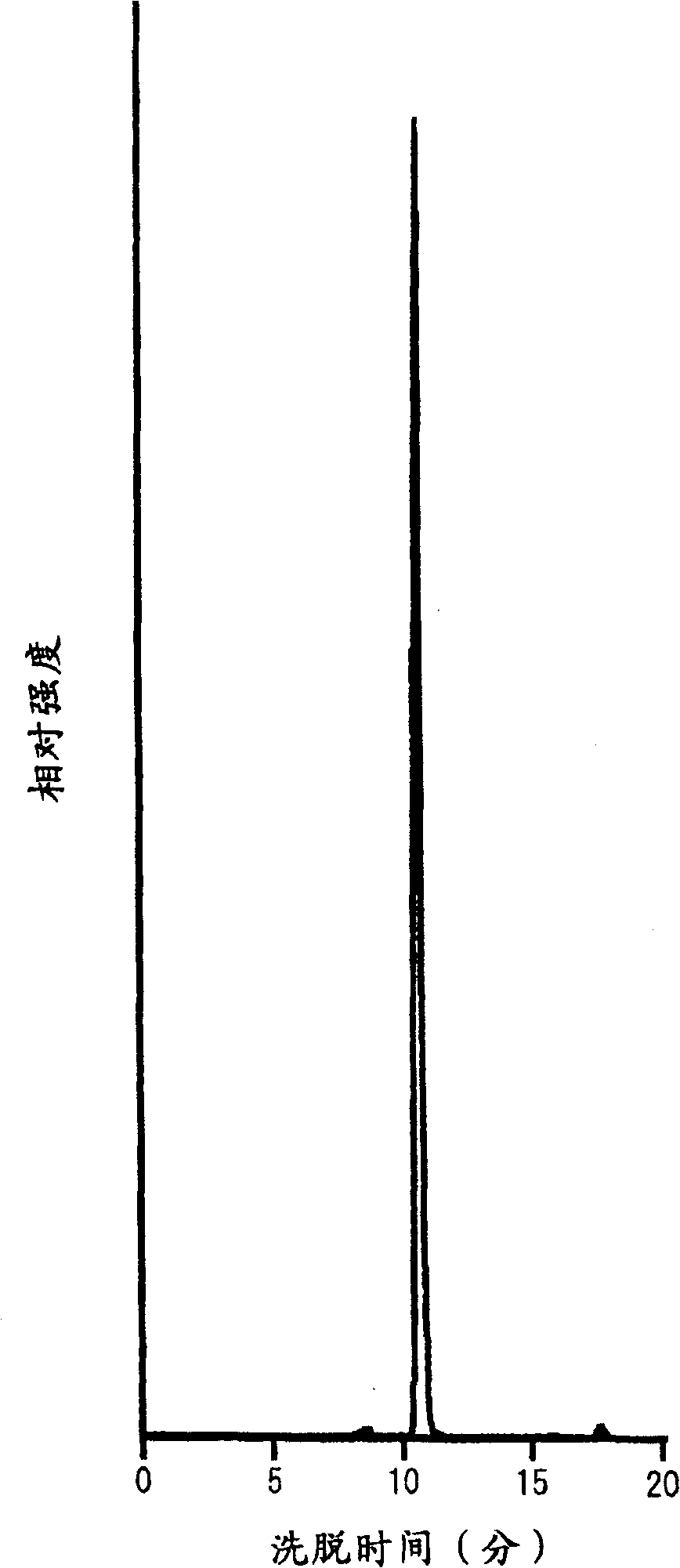
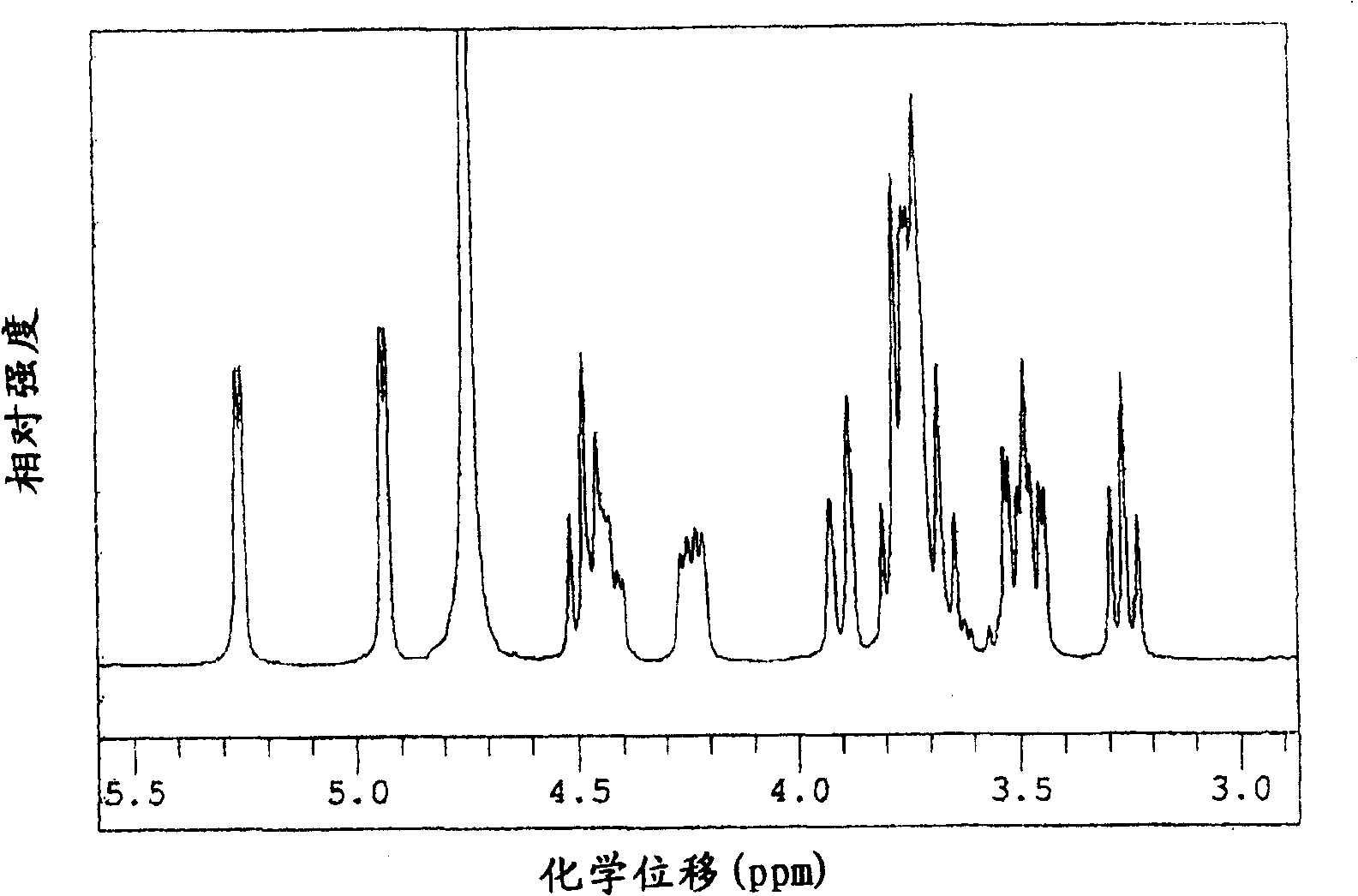
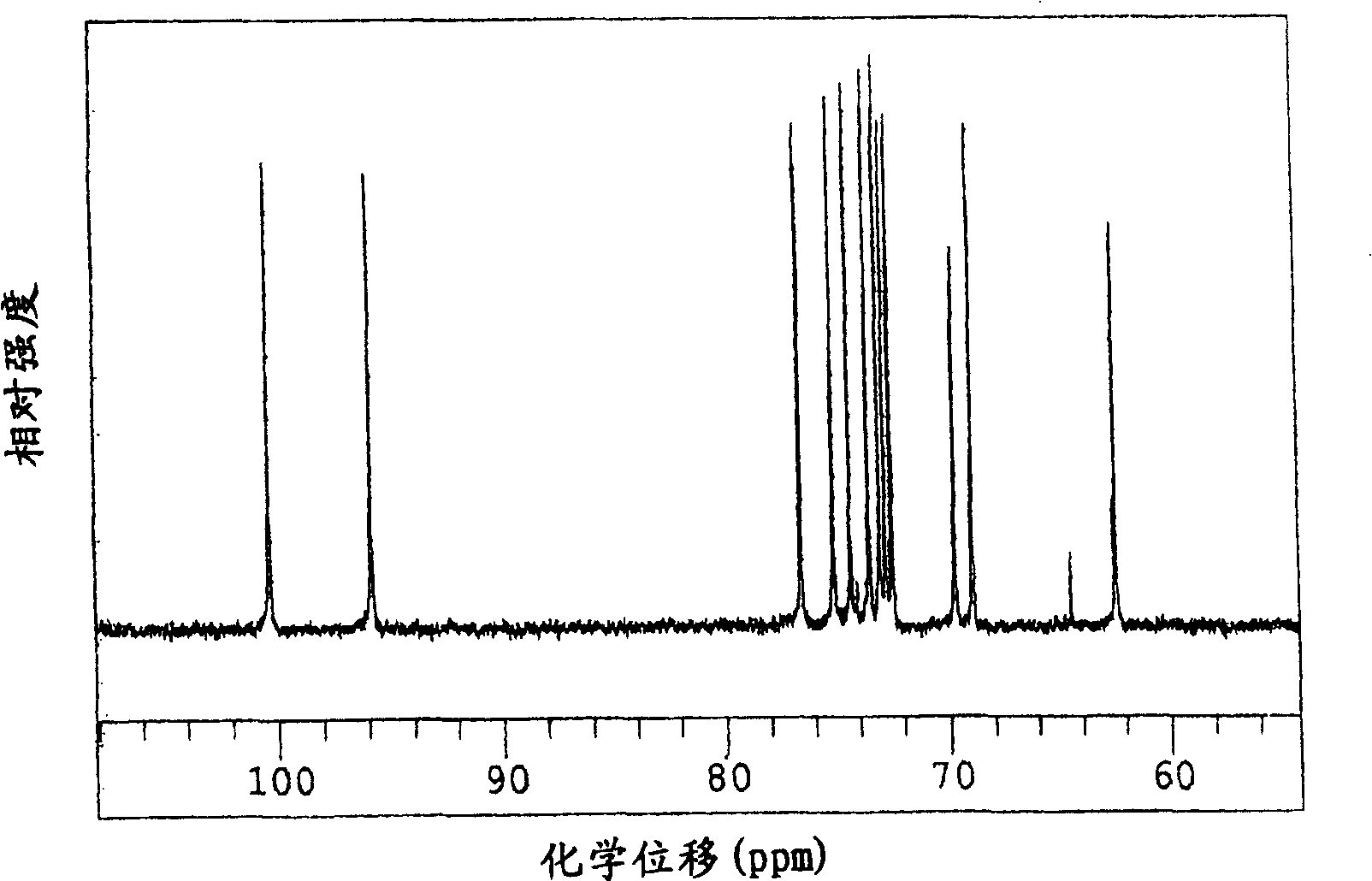
[0304] Make potato starch milk with a concentration of about 1% by mass starch milk, then add calcium chloride so that the final concentration is 1mM, adjust to pH 6.0, heat at 95°C for about 20 minutes to gelatinize, and then cool to about 40°C , according to the ratio of 0.26ml (about 1 unit) per 1 gram of starch solids, add the concentrated enzyme solution containing cyclic maltosyl maltose synthase obtained by the method of Example 1, and react at pH 6.0 and temperature 40°C for 48 Hour. Heat the reaction solution to 95°C, keep it for 30 minutes and then cool it down. The filtrate obtained after filtration is decolorized with activated carbon according to the conventional method, and desalted, purified and concentrated by H-type and OH-type ion exchange resins. The yield was about 90%, and the syrup containing cyclic maltosyl maltose with a density|concentration of 65 mass % was obtained. This product contains 31.4% by mass of cyclic maltose-based maltose, 2.2% by mass of...
Embodiment 3
[0306] Tapioca starch is made into starch milk with a concentration of about 1% by mass, then calcium carbonate is added according to a final concentration of 0.1% by mass, adjusted to pH6.0, and then α-amylase ( Manufactured by Nobo Corporation under the trade name "Tamamil 60L", reacted at 95°C for 10 minutes, then autoclaved at 120°C for 20 minutes, and then rapidly cooled to about 40°C to obtain a liquefied solution with a DE of about 3. The liquefaction solution is added according to 1 gram of starch solids 0.26ml (about 1 unit) with the concentrated solution containing cyclic maltosyl maltose synthase obtained by the method of Example 1 and isoamylase ( Hayashibara Biochemical Research Institute Co., Ltd.), reacted for 48 hours at pH 6.0 and 40°C. The reaction solution was heated to 95°C and kept for 30 minutes, then cooled, and the filtered filtrate was used in a conventional method. Activated carbon decolorizes, purifies after desalination by H type and OH type ion exc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com