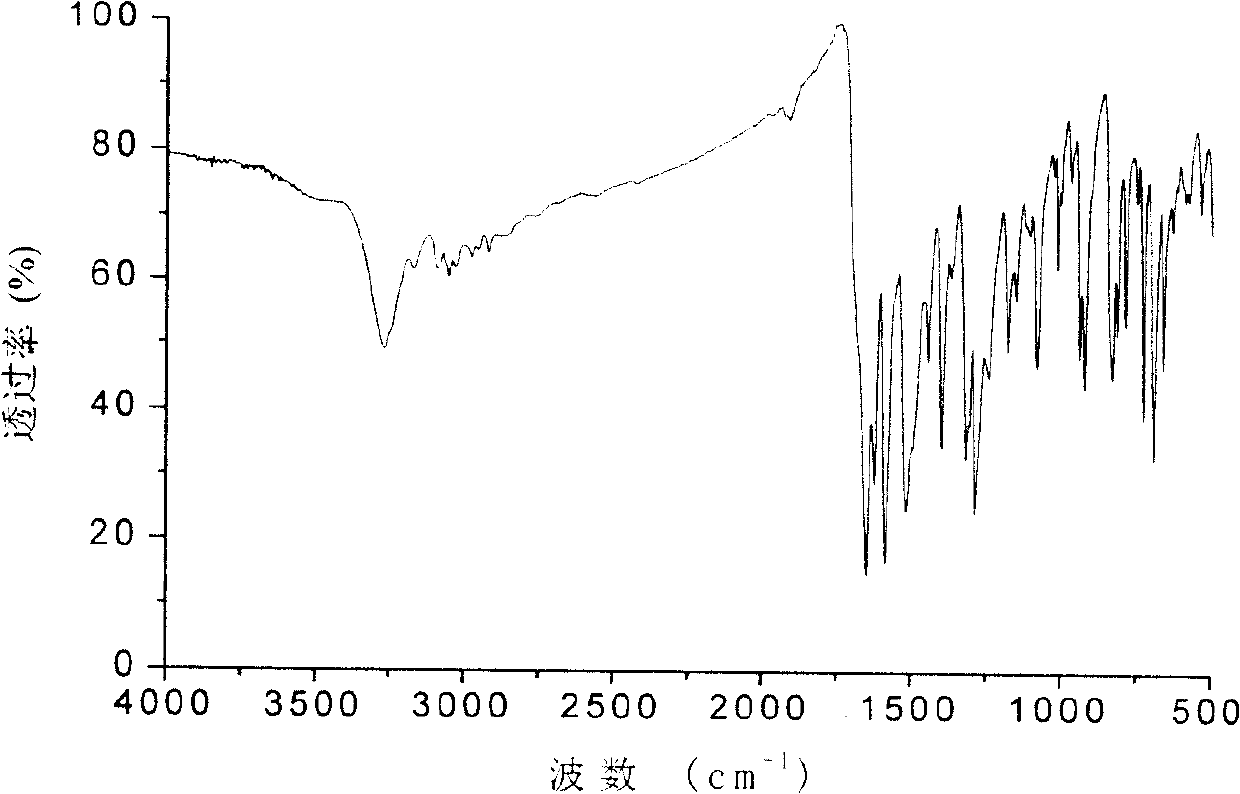
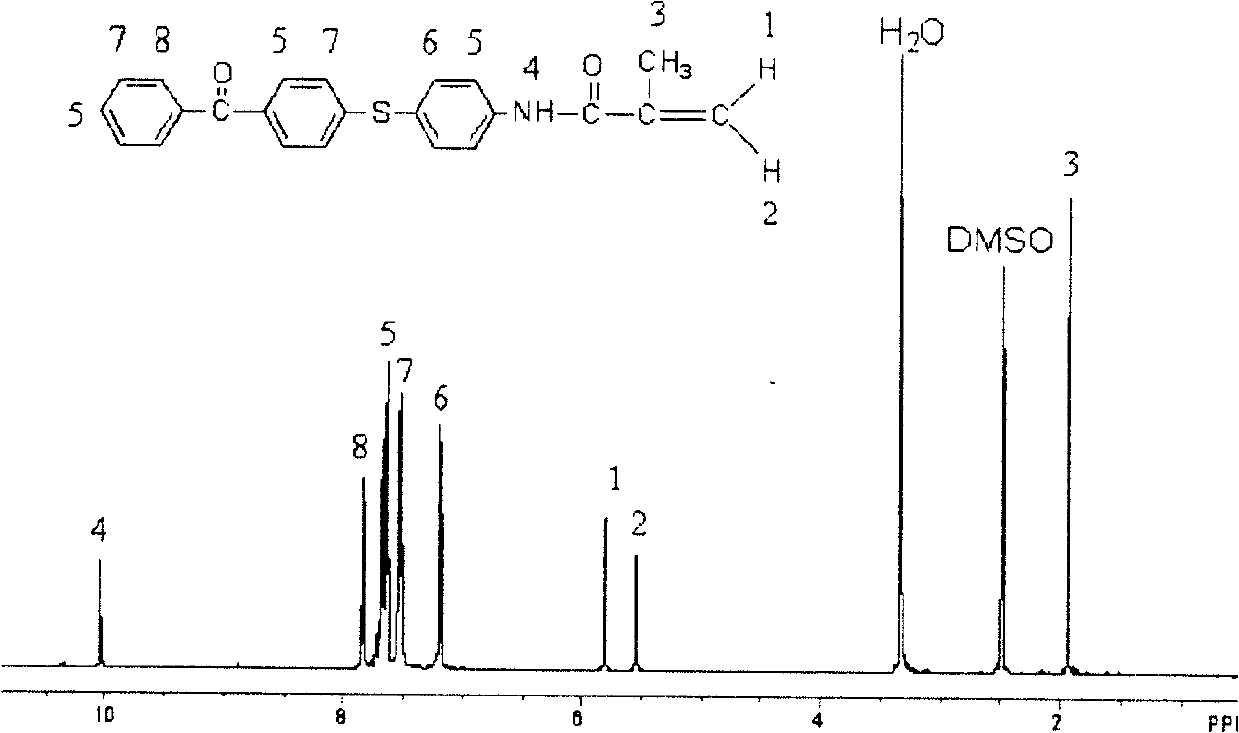
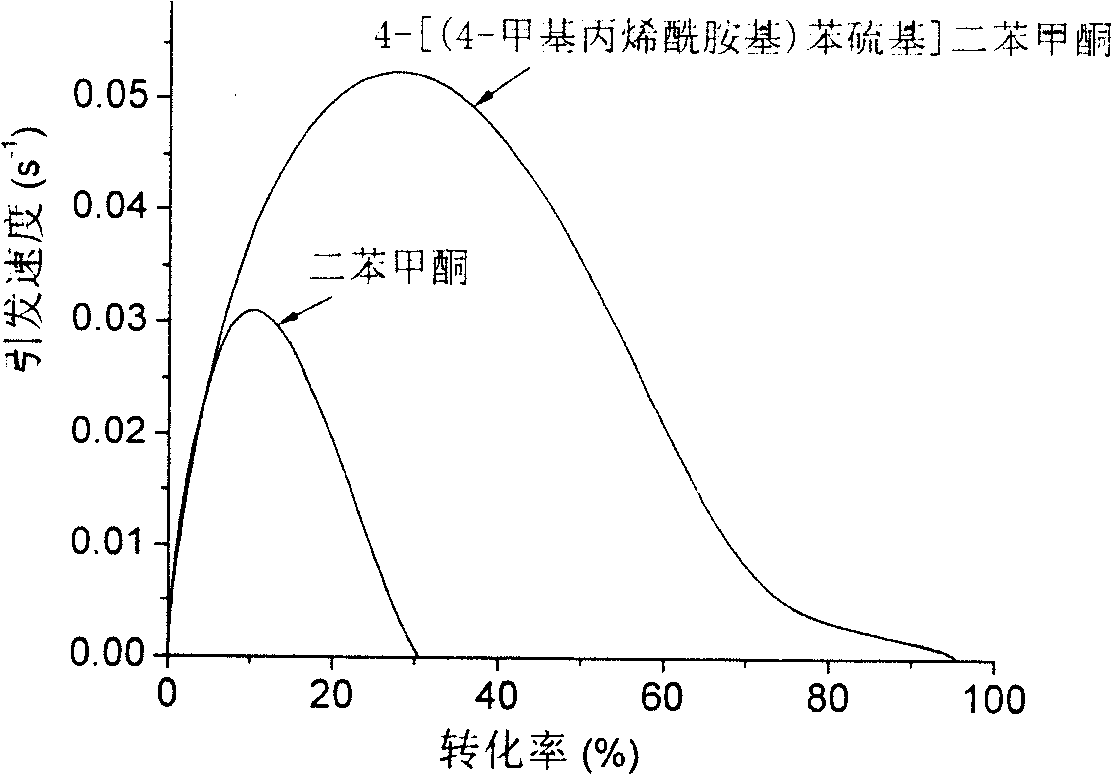
Polymerisable benzophenone photoinitiator and its preparing method
A technology of benzophenone and photoinitiator, which is applied in the field of benzophenone photoinitiator and its preparation, can solve problems such as easy volatilization, migration, and reduced photopolymerization initiation efficiency, so as to improve compatibility, initiate Increased speed, reduced toxicity and migration effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (a) In a three-necked flask equipped with a water separator, a condenser and a nitrogen protection device, under nitrogen protection, add 7.50 grams of p-aminothiophenol, 50 mL of 1-methyl-2-pyrrolidone, and 3.60 grams of potassium hydroxide , 12.96 grams of 4-chlorobenzophenone and 30 mL of toluene, stirred to make the system evenly mixed. Slowly raise the temperature to 130-135°C in an oil bath, and react for 3 hours, during which the water in the system azeotropes out with toluene. Slowly raise the temperature to 170-175°C again, and react for 3 hours. After slowly cooling down to room temperature, filter with suction to remove insoluble matter. The filtrate was slowly added to a vigorously stirred mixture of 100 mL of concentrated hydrochloric acid and 500 mL of ice-water within 1 hour.
[0028] Suction filtration, washing with water and petroleum ether, and drying the filter cake in a vacuum oven to obtain 18.35 g of product. Recrystallize from a mixed solution ...
Embodiment 2
[0032] (a) In a three-necked flask equipped with a water separator, condenser and nitrogen protection device, under nitrogen protection, add 8.80 grams of p-aminothiophenol, 40 mL of 1-methyl-2-pyrrolidone, and 2.60 grams of potassium hydroxide , 30 mL of toluene and 8.80 grams of 4,4' dichlorobenzophenone. Slowly raise the temperature to 130-135°C within 30 minutes, and react for 3 hours, during which water azeotropes out with toluene. Slowly raise the temperature again so that the temperature of the system rises to between 170-175° C., all the toluene is released, and the reaction is carried out for 3 hours. Cool down to room temperature naturally, and remove the insoluble matter by suction filtration. Slowly pour the filtrate into a mixture of 140mL concentrated hydrochloric acid and 400mL ice-water, stir, filter with suction, and wash with water. Vacuum drying gave 16.01 g of dark yellow to light brown 4,4'-bis[(4-amino)phenylthio]benzophenone hydrochloride. Heat and di...
Embodiment 3
[0036] (a) In a three-necked flask equipped with a water separator, a condenser, and a nitrogen protection device, add 4.40 grams of p-aminothiophenol, 40 mL of 1-methyl-2-pyrrolidone, and 2.60 grams of potassium hydroxide in sequence under nitrogen protection. , 30 mL of toluene and 8.80 grams of 4,4'-dichlorobenzophenone. Slowly raise the temperature to 130-135°C within 30 minutes, and react for 3 hours, during which water azeotropes out with toluene. Slowly raise the temperature again so that the temperature of the system rises to between 170-175° C., all the toluene is released, and the reaction is carried out for 3 hours. Cool down to room temperature naturally, and remove the insoluble matter by suction filtration. Slowly pour the filtrate into a mixture of 75mL of concentrated hydrochloric acid and 250mL of ice-water, stir, filter with suction, and wash with water. Vacuum drying to obtain 12.71 g of dark yellow to light brown 4-chloro-4'-[(4-amino)phenylthio]benzophen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com