A culturing method of human embryonic stem cells and a special culturing medium thereof
A technology for embryonic stem cells and culture medium, applied in the field of cell culture method and special culture medium, can solve the problems of complicated operation, pollution, transplantation failure, etc., and achieve the effects of broad application prospect, long passage time and high safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, the acquisition of special medium for human embryonic stem cells
[0038] 1. Routine culture of human embryonic stem cells
[0039] Reagent:
[0040] PBS: weigh 8g NaCl, 0.2g KCl, 1.44g Na 2 HPO 4 and 0.24g KH 2 PO 4 , plus ddH 2 O was adjusted to 1000 mL, and the pH value of the solution was adjusted to 7.4 with HCl.
[0041] 200mM glutamine storage solution (200×): Weigh 0.292g glutamine, dissolve it in 10mL PBS, filter and sterilize, aliquot and store in a -70°C refrigerator.
[0042] 2M β-mercaptoethanol (20000×): Take 1mL of 14.3M β-mercaptoethanol, add 6.15mL PBS to dilute, and filter to sterilize.
[0043] Human embryonic stem cell medium (HESM): 20% serum replacement (Knock-out Serum Replacement, KSR), 1mM glutamine, 0.1mM β-mercaptoethanol, 1% non-essential amino acids (Non-essentialAminoAcids) (Gibco, USA), 4ng / mL basic fibroblast growth factor (bFGF) with ddH 2 O was adjusted to 1000mL.
[0044] 0.5 mg / mL Dispase: Weigh 10 mg of Dispas...
Embodiment 2
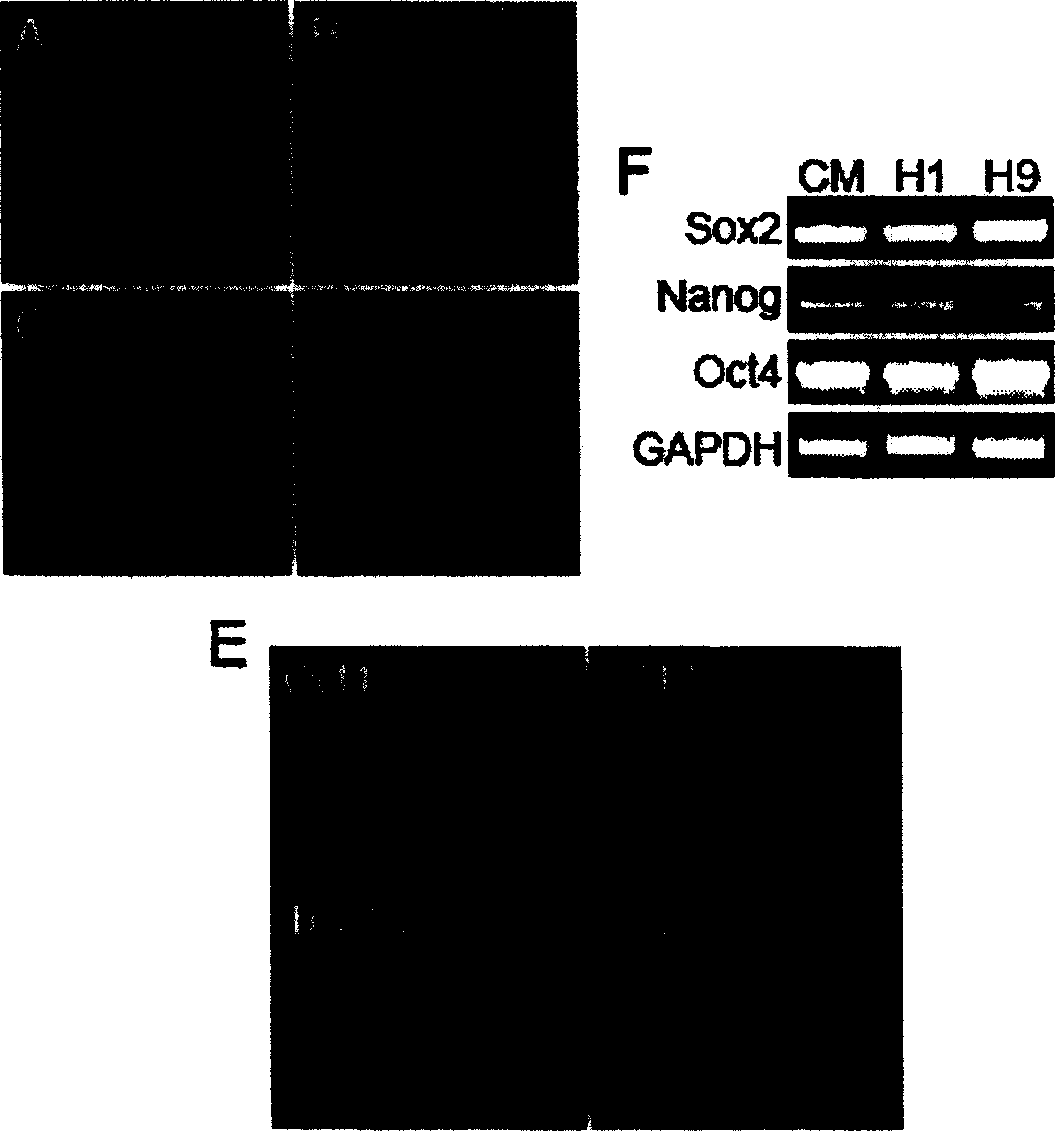
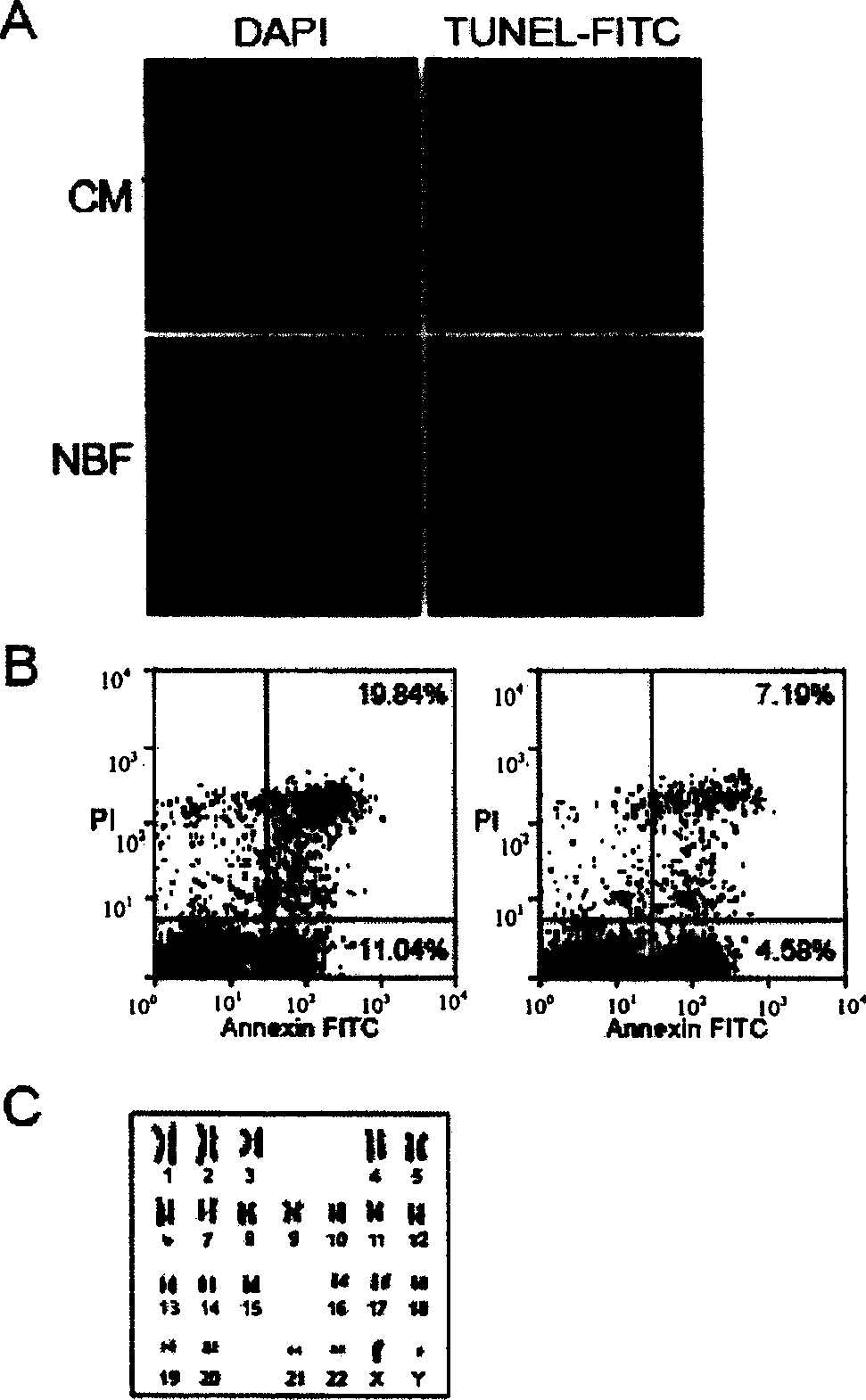
[0091] Example 2, Determination of Human Embryonic Stem Cell Culture Method and Detection of Cultured Cells
[0092] 1. Determination of the culture method of human embryonic stem cells
[0093] Although human embryonic stem cells can be cultured for a long time with the culture medium of the present invention, it has not achieved complete component determination and no animal source, because the matrigel that coats the culture dish is derived from the osteosarcoma of mice, so in order to make the culture system complete To achieve no animal source, four kinds of human matrix (fibronectin, collagen IV, vitaminectin, laminin) and their different combinations are used to coat the culture dish to detect the status of human embryonic stem cells in these culture systems. The coating method is as follows:
[0094] 1) Put the human matrix fibronectin, collagen IV, vitaminectin and laminin purchased from Sigma in the United States at 4°C for 12-24 hours to thaw, and then divide them t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com