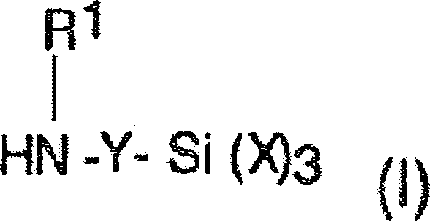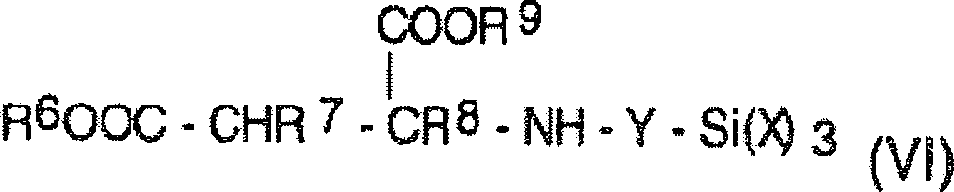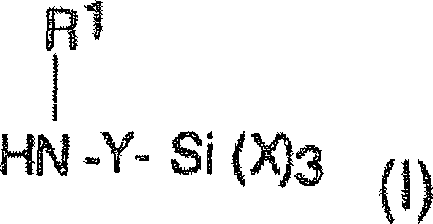Silante terminated polyurethane
A silane-terminated, polyurethane technology, applied in the field of alkoxysilane-functional urethane compositions, can solve the problems of foaming, inability to provide sufficient tensile strength and/or elongation at break, and easy film cracking.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] This example illustrates the preparation of silane-functional aspartic acid esters according to the invention. Aspartate resins were prepared according to US Patent No. 4,364,955 to Kramer et al. Add 1483 grams (8.27 equivalents) of 3-aminopropyltrimethoxysilane to a 5-liter flask equipped with a stirrer, thermocouple, nitrogen inlet, addition funnel, and condenser, then within two hours, add 1423.2 g (8.27 equivalents) of diethyl maleate, and maintained at this temperature for 5 hours. The unsaturation value by iodine titration was 0.6, indicating that the reaction was nearly 99% complete. use Viscometer (Model DV-II+, Brookfield Engineering, Inc., Middleboro, MA, spindle 52) with a viscosity of 11 centipoise measured at 25°C at 100 rpm.
Embodiment 2
[0133] This example describes the preparation of a silane-terminated polyurethane (STP1) according to the invention. In a 5-liter round bottom flask equipped with a stirrer, nitrogen inlet, addition funnel and condenser tube, add 150.9 grams (1.1 equivalents) of isophorone diisocyanate, 3664.1 grams (0.6 equivalents) as described in U.S. Patent No. 4,355,188 The prepared equivalent weight is 6411 polyether monohydric alcohol and 0.6 gram of dibutyltin dilaurate. The reaction was heated at 60° C. for 3 hours, after which time the NCO content was determined by NCO titration to be 0.65% by weight (theoretical = 63%). Then 202.2 g (0.57 equivalents) of the silane-functional aspartic ester of Example 1 were added and the mixture was maintained at 60° C. for 60 minutes, after which time no NCO groups could be detected by IR spectroscopy. At this point, 20 grams of vinyltrimethoxysilane was added as a desiccant. The viscosity at 25°C is 16100 centipoise.
Embodiment 3
[0135] This example describes the preparation of a silane-terminated polyurethane (STP2) according to the invention. To a 5 L round bottom flask equipped with a stirrer, nitrogen inlet, addition funnel and condenser was charged 366.7 g (3.3 eq) of isophorone diisocyanate, 122.7 g (0.165 eq) of n-butanol and 0.2 g of dilauric acid Dibutyltin. The reaction was heated at 60° C. for 3 hours, after which time the NCO content was determined to be 14% by weight by NCO titration (theoretical = 14.2%). Then 605.1 g (1.65 equivalents) of the silane-functional aspartic acid ester of Example 1 were added and the mixture was maintained at 60° C. for 60 minutes, after which time no NCO groups could be detected by IR spectroscopy. At this point, 5.5 grams of vinyltrimethoxysilane was added as a desiccant. The viscosity at 25°C is 242000 centipoise.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com