Use of human lysozyme in preparing medicines for cutaneous pruritus
A technology of human lysozyme and pruritus, which is applied in the field of application of human lysozyme in the preparation of medicines for treating pruritus, and can solve problems such as large side effects, impact on health, skin burning, tingling sensation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
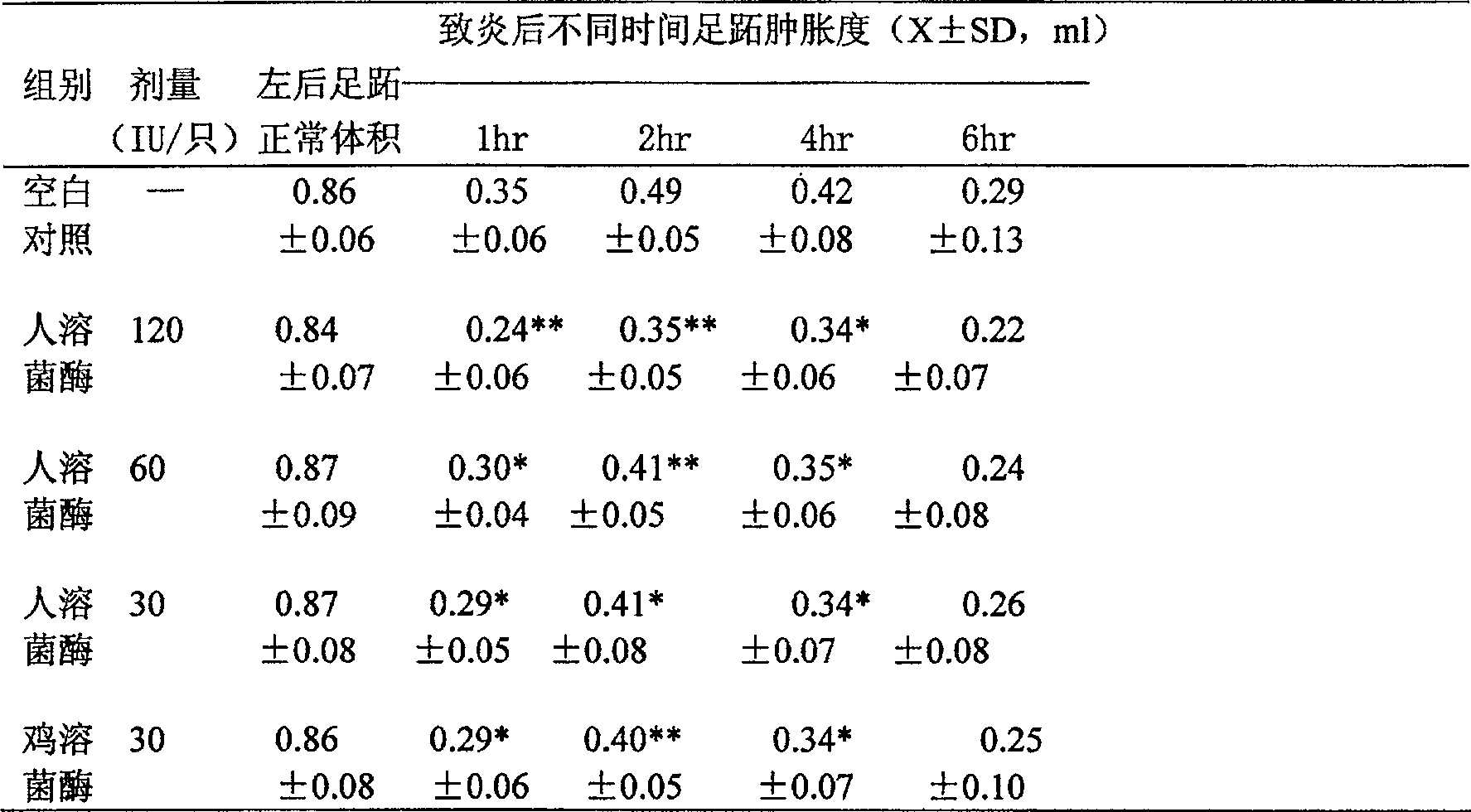
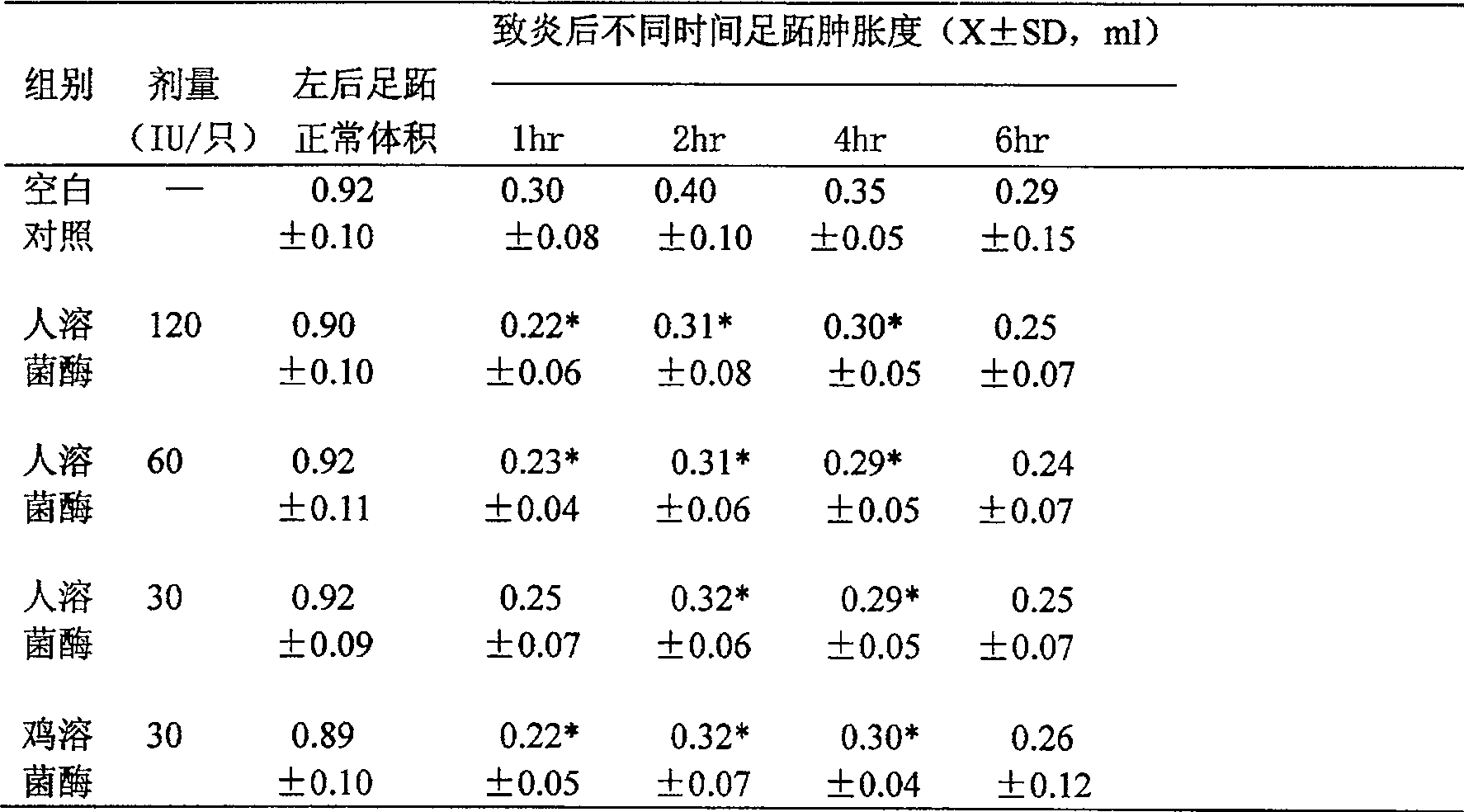
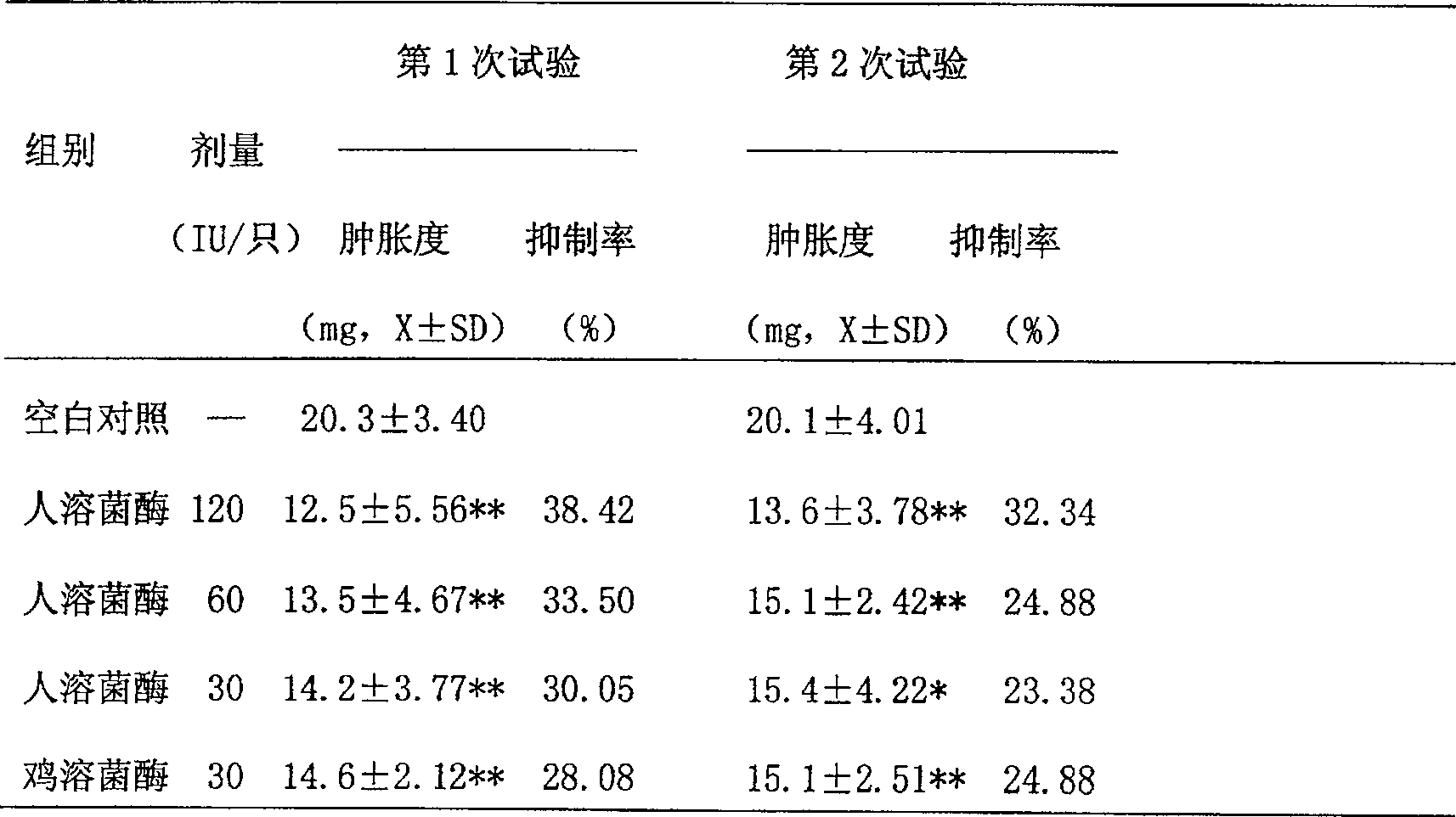
[0065] Recombinant human lysozyme is based on the preparation of 200 ml medium, with 6 ml phosphoric acid, 3 g magnesium sulfate, 4 g potassium sulfate, 1 g potassium hydroxide, 1.5 g calcium sulfate, add distilled water to 200 ml, after autoclaving Glycerol tube seeds were inoculated, the rotation speed of the shaker was 250 revolutions per minute, the cultivation temperature was 20° C., and the cultivation was carried out on a constant temperature bed for 36 hours. The seed tank culture is carried out, and finally the production tank culture is carried out. Extracting and purifying the culture medium after fermentation and expression, freeze-drying the extracted and purified gene recombined human lysozyme concentrate, measuring protein quantity, purity and preservation of lysozyme activity.
[0066] The recombinant human lysozyme with a purity of 95% was prepared to 1500U / ml, phosphate buffer 10-20mM (pH6.5-7.5) 80%, 15% propylene glycol, 1% water-soluble azone, 5 / 10,000 Twe...
Embodiment 2
[0068] According to the method described in Example 1, the recombinant human lysozyme was obtained, and the recombinant human lysozyme with a purity of 97% was obtained at 30000 U / ml, and the phosphate buffer was 10-20 mM (pH6.5-7.5) 84%, 13% Propylene glycol, 2% water-soluble azone, and 5 / 10,000 Tween 80 are mixed homogeneously at room temperature, and drops are prepared according to pharmaceutical regulations in a pharmaceutical factory meeting GMP requirements.
Embodiment 3
[0070] According to the method described in Example 1, the recombinant human lysozyme was obtained, and the recombinant human lysozyme with a purity of 98% was obtained at 100,000 U / ml, and the phosphate buffer was 10-20 mM (pH6.5-7.5) 78%, 21% Propylene glycol, 1% water-soluble azone, and 5 / 10,000 Tween 80 are mixed homogeneously at room temperature, and are made into drops according to pharmaceutical regulations in a pharmaceutical factory meeting GMP requirements.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com