Myricetin dispersion tablets for treating cardio-cerebral blood vessel diseases and preparation method thereof
A technology of dispersible tablets and myricetin, which is applied to cardiovascular system diseases, blood diseases, metabolic diseases, etc., can solve the problems of affecting the efficacy of tablets, reducing bioavailability, and affecting the absorption of main ingredients, so as to achieve good prevention and treatment effects , Improve the absorption rate in the body and improve the solubility of the main drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Myricetin 100g
[0021] Cross-linked polyvinylpyrrolidone 13g (internal addition)
[0022] 4g (additional)
[0023] Polyvinylpyrrolidone 15g
[0024] Microcrystalline Cellulose 57g
[0025] α-Lactose 57g
[0026] Sodium Lauryl Sulfate 5g (additional)
[0027] Magnesium stearate 1.3g (additional)
[0028] Appropriate amount of essence (additional)
[0029]
[0030] A total of 1000 pieces
[0031] Weigh the drug and the excipients after passing through the 100-mesh sieve according to the prescription amount, fully mix the drug and the internally added excipients with the equal-volume incremental method, and grind. Add the prepared 10% polyvinylpyrrolidone aqueous solution, fully grind to make soft material. Pass the prepared soft material through a 50-mesh sieve to make wet granules, and then dry it in an oven at 60°C for 2 hours. After taking it out, pass it through a 50-mesh sieve for granul...
Embodiment 2
[0036] Myricetin 100g
[0037] ice flakes 2g
[0038] Cross-linked polyvinylpyrrolidone 12g (internal addition)
[0039] 4g (additional)
[0040] Polyvinylpyrrolidone 15g
[0041] Microcrystalline Cellulose 58g
[0042] Pregelatinized starch 57g
[0043] Sodium Lauryl Sulfate 4g (additional)
[0044] Magnesium stearate 1.2g (additional)
[0045] Appropriate amount of essence (additional)
[0046]
[0047] A total of 1000 pieces
[0048] Its preparation process and method are the same as in Example 1.
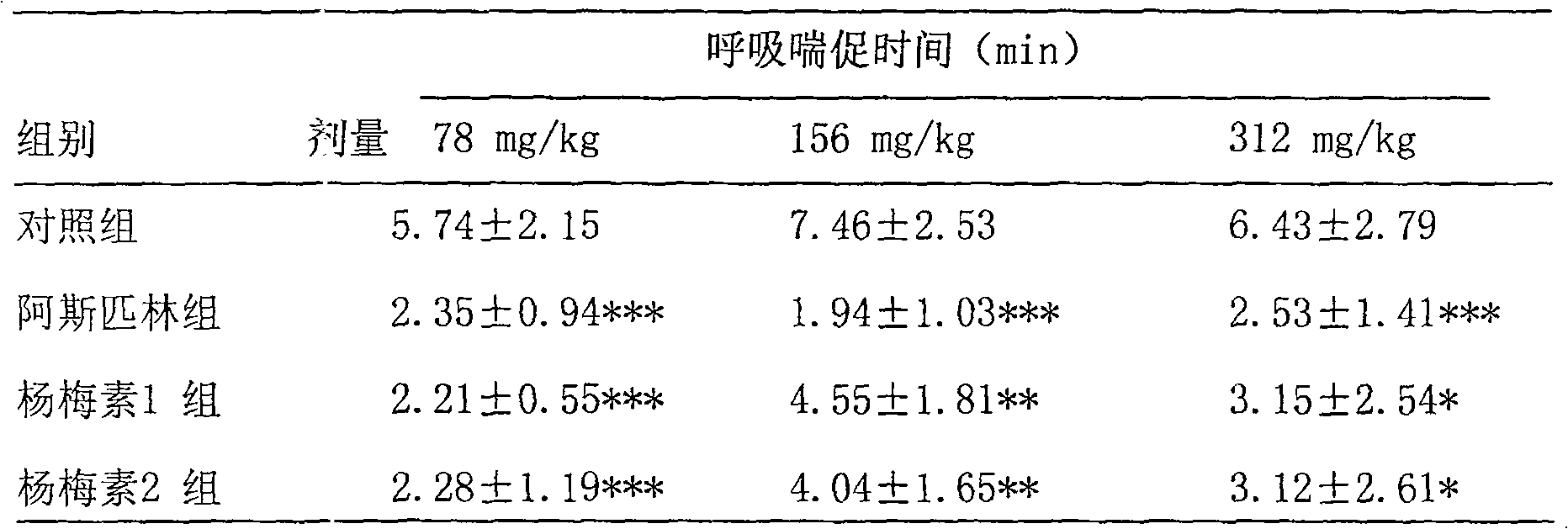
[0049] Each tablet of this product weighs about 250mg, contains myricetin 100mg / tablet, and borneol 2mg / tablet. The appearance is yellow-green, uniform in color, and all indicators are in line with the relevant regulations of the 2005 edition of the "Chinese Pharmacopoeia".
[0050] Usage and dosage: Take orally, 1-2 tablets each time, 3 times a day.
[0051] Storage: sealed, in a cool dry place.
Embodiment 3
[0053] Myricetin 200g
[0054] Polyvinylpyrrolidone 35g
[0055] Microcrystalline Cellulose 150g
[0056] 130g pregelatinized starch
[0057] Sodium Lauryl Sulfate 10g (additional)
[0058] Magnesium stearate 2.5g (additional)
[0059] Appropriate amount of essence (additional)
[0060]
[0061] A total of 1000 pieces
[0062] Its preparation process and method are the same as in Example 1.
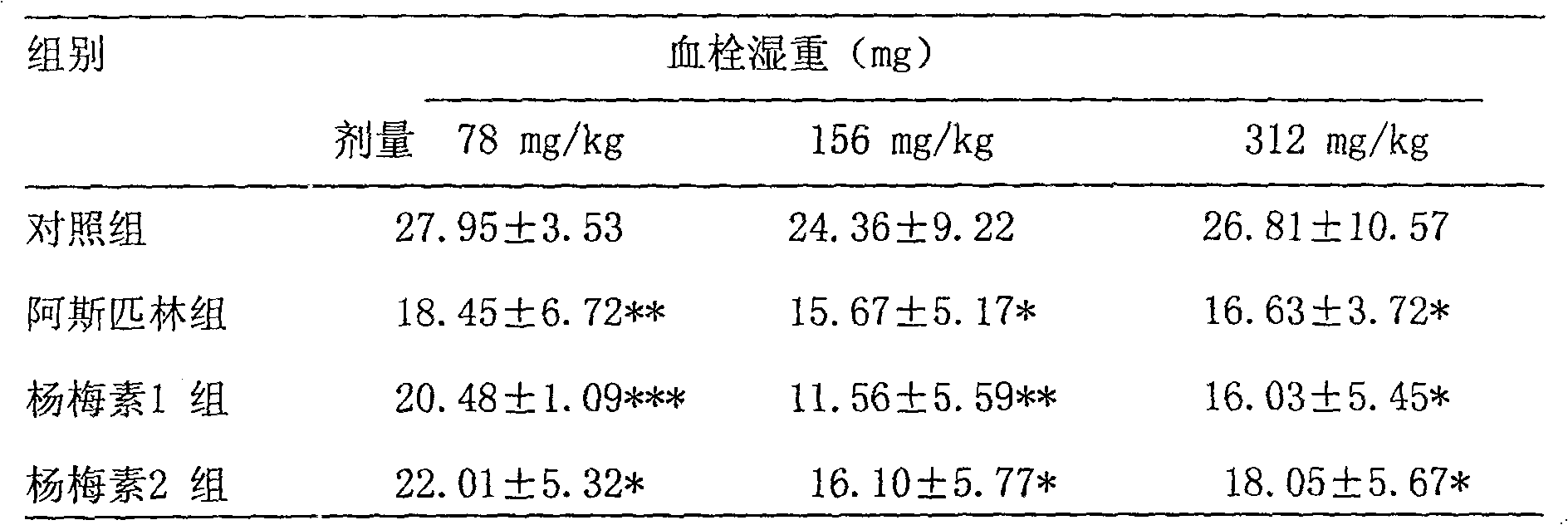
[0063] Each tablet of this product weighs about 0.5g and contains 200mg myricetin per tablet. The appearance is yellow-green, uniform in color, and all indicators are in line with the relevant regulations of the 2005 edition of the "Chinese Pharmacopoeia".
[0064] Usage and dosage: Take orally, 1 tablet each time, 3 times a day.
[0065] Storage: sealed, in a cool dry place.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com