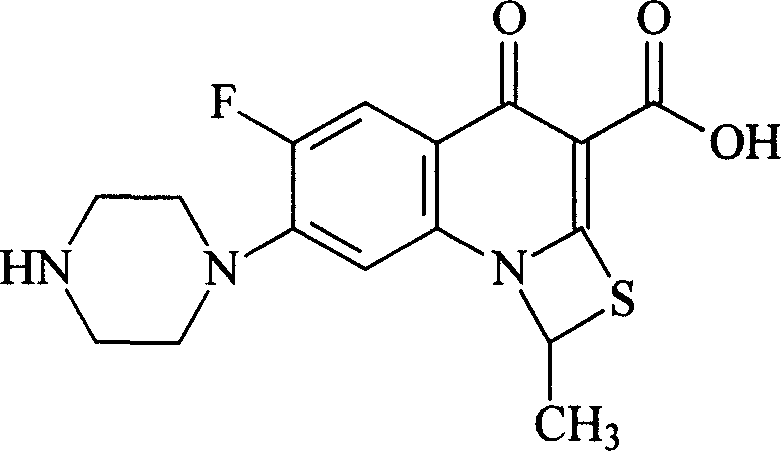
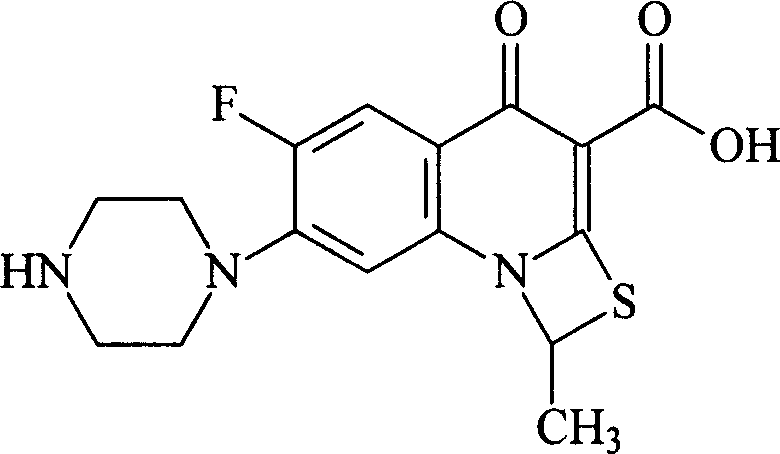
Anti-inflammatory and sterilizing preparation
An anti-inflammatory, sterilizing and preparation technology, applied in the field of pharmaceutical preparations, to achieve the effects of low production cost, suitable for storage and transportation, and stable products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Prescription: 3g of compound II, 5.5ml of acetic acid, appropriate amount of 5% sodium hydroxide, 0.9g of sodium chloride, 0.3g of methylparaben and 0.1g of propylparaben, add appropriate amount of water for injection to make eye drops 1000ml, The specification of eye drops is 3mg / ml.
[0061] Preparation: at 10-15°C, add 3g of compound II to 400ml of water for injection, add 5.5ml of acetic acid, stir for 60 minutes, add 5% sodium hydroxide solution to adjust the pH of the solution to 6.2, then add 0.9g of sodium chloride, and stir until Dissolve; in addition, take 0.3g of methylparaben and 0.1g of propylparaben, add 200ml of boiling distilled water to dissolve them; mix the above two solutions, add distilled water to a total of 1000ml, stir well, and use 0.22μ micro Filter through a pore filter, fill, seal tightly, sterilize at 100°C for 30 minutes with circulating steam, and pack in separate packages. Note: Compound II was supplied by a Beijing Chemical Technology C...
Embodiment 2
[0063] Prescription: 4g compound II, 7.5ml acetic acid, appropriate amount of 5% sodium hydroxide, 0.9g sodium chloride, 0.3g methylparaben and 0.1g propylparaben, add appropriate amount of water for injection to make eye drops 1000ml, The specification of eye drops is 4mg / ml.
[0064] Preparation: at 10-15°C, add Compound II to 700ml of water for injection, add 7.5ml of acetic acid, stir for 60 minutes, add 5% sodium hydroxide solution to adjust the pH of the solution to 6.5, then add 0.9g of sodium chloride, and stir until dissolved ;In addition, take 0.3g of methylparaben and 0.1g of propylparaben, add 200ml of boiling distilled water to dissolve them; mix the above two solutions, add distilled water to a total of 1000ml, stir well, and use a 0.22μ microporous Membrane filtration, filling, sealing tightly, sterilizing at 100°C for 30 minutes with flowing steam, subpackaging, ready to use.
Embodiment 3
[0066] Prescription: Compound II hydrochloride 2.21g, acetic acid 1.0ml, appropriate amount of 5% sodium hydroxide, sodium chloride 0.9g, methylparaben 0.3g and propylparaben 0.1g, add appropriate amount of water for injection to prepare Eye drops 1000ml, the specification of eye drops is 2.2mg / ml.
[0067] Preparation: at 10-15°C, add the hydrochloride of Compound II (prepared according to Example 7) to 500ml of water for injection, add 1.0ml of acetic acid, stir for 30 minutes, add 5% sodium hydroxide solution to adjust the pH of the solution to 5.8, Then add 0.9g of sodium chloride and stir until dissolved; in addition, take 0.3g of methylparaben and 0.1g of propylparaben, add 200ml of boiling distilled water to dissolve them; mix the above two solutions, add distilled water to the total Make 1000ml, stir well, filter with 0.22μ microporous membrane, fill, seal tightly, sterilize at 100°C for 30 minutes with circulating steam, and pack separately to get ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com