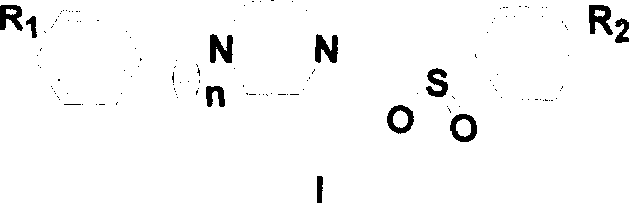
N,N'-disubstituted piperazine derivative, and its preparation method and medicinal composition and uses
A di-substituted and derivative technology, applied in the direction of drug combination, pharmaceutical formulation, medical preparation containing active ingredients, etc., can solve the problems of Shaker channel selectivity loss, ion selectivity influence, channel loss selectivity, etc. Abundant, low toxicity, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Computer Virtual Screening :
[0063] In April 1998, Doyle published the crystal structure of the first ion-selective channel in Science, which was derived from the potassium ion channel of the bacterium Streptomyces lividans. Using the crystal structure of the potassium channel, as well as the information on the action sites of quaternary ammonium ion (QA), 4-aminoquinoline, and toxins such as scorpion venom, snake venom, and honeybee venom on the potassium channel, two active sites of the potassium channel were selected, and the molecular The docking program DOCK4.0 carried out a three-dimensional database search on them to find possible blockers. The small molecule three-dimensional structure database searched is the ACD-3D small molecule database, which has about 250,000 compounds.
[0064] experimental method:
[0065] (1) Structure processing of small molecule compounds
[0066] Before the three-dimensional structure of small molecules can be used for molecu...
Embodiment 2
[0088] Preparation of 1-benzyl-4-[2-(4-chloro-benzenesulfonyl)-ethyl]-piperazine (Ia)
[0089]
[0090] At normal temperature, feed freshly prepared dry HCl gas into a 200ml three-necked flask containing 8.6g (0.1mol) anhydrous piperazine dissolved in 50ml THF. Under vigorous stirring, react for 6h, remove HCl gas, add 8.6g (0.1mol) of anhydrous piperazine, react at room temperature for 2h, then reflux for 6h under vigorous stirring. Cool, filter and wash the filter cake with cold THF. After drying, 22.4 g of white crystals of piperazine monohydrochloride were obtained (92.3% yield).
[0091] Put 4.86g (0.02mol) of piperazine monohydrochloride and 20ml of THF into a 100ml eggplant-shaped bottle. At 0-5°C, 2.28 g (0.018 mol) benzyl chloride dissolved in 20 ml THF was added dropwise, and the drop was completed after 1 h. Reacted overnight at room temperature, refluxed for 2 hours, and evaporated the solvent. Then, add 30ml of water, adjust the pH=8-9 with saturated potass...
Embodiment 3
[0098] Preparation of 1-(4-fluorophenyl)-4-[2-(4-chloro-benzenesulfonyl)-ethyl]-piperazine (Ib)
[0099] Replace 1-benzylpiperazine with 1-(4-fluorophenyl)-piperazine, operate as described in Example 2, all the other required raw materials, reagents and preparation methods are the same as Example 2, to obtain target compound 1-( 4-fluorophenyl)-4-[2-(4-chloro-phenylsulfonyl)-ethyl]-piperazine.
[0100] IR (KBr): 1625cm -1 . 1 HNMR (CDCl 3 )ppm: 2.55(t, 4H,); 2.85(t, 2H); 2.98(t, 4H); 3.35(t, 2H); 6.82(m, 2H); 6.94(m, 2H); 2H); 7.87 (m, 2H). HRMS(SCI)m / z calcd for M + , 382.0918; found, 382.0922.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com