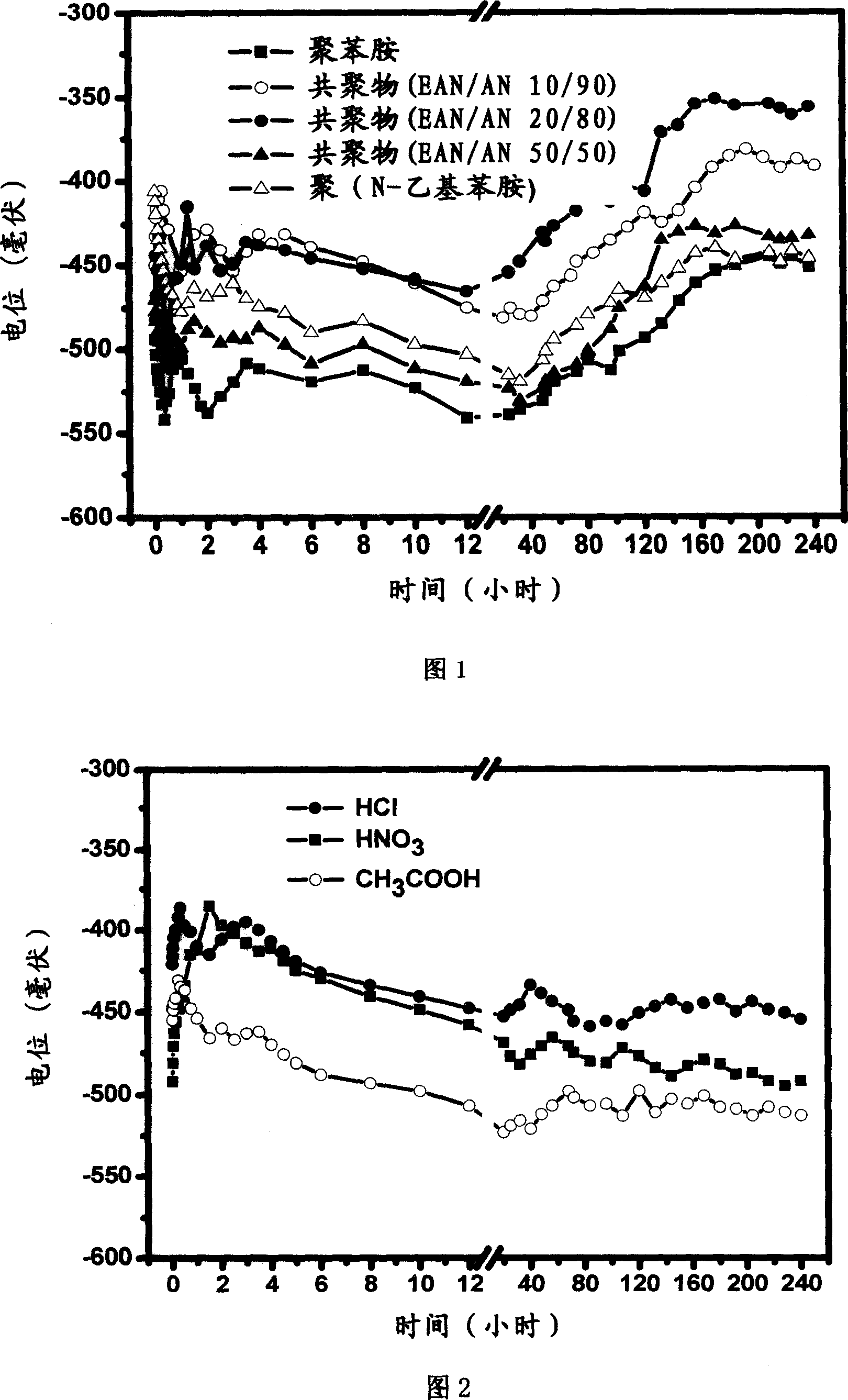
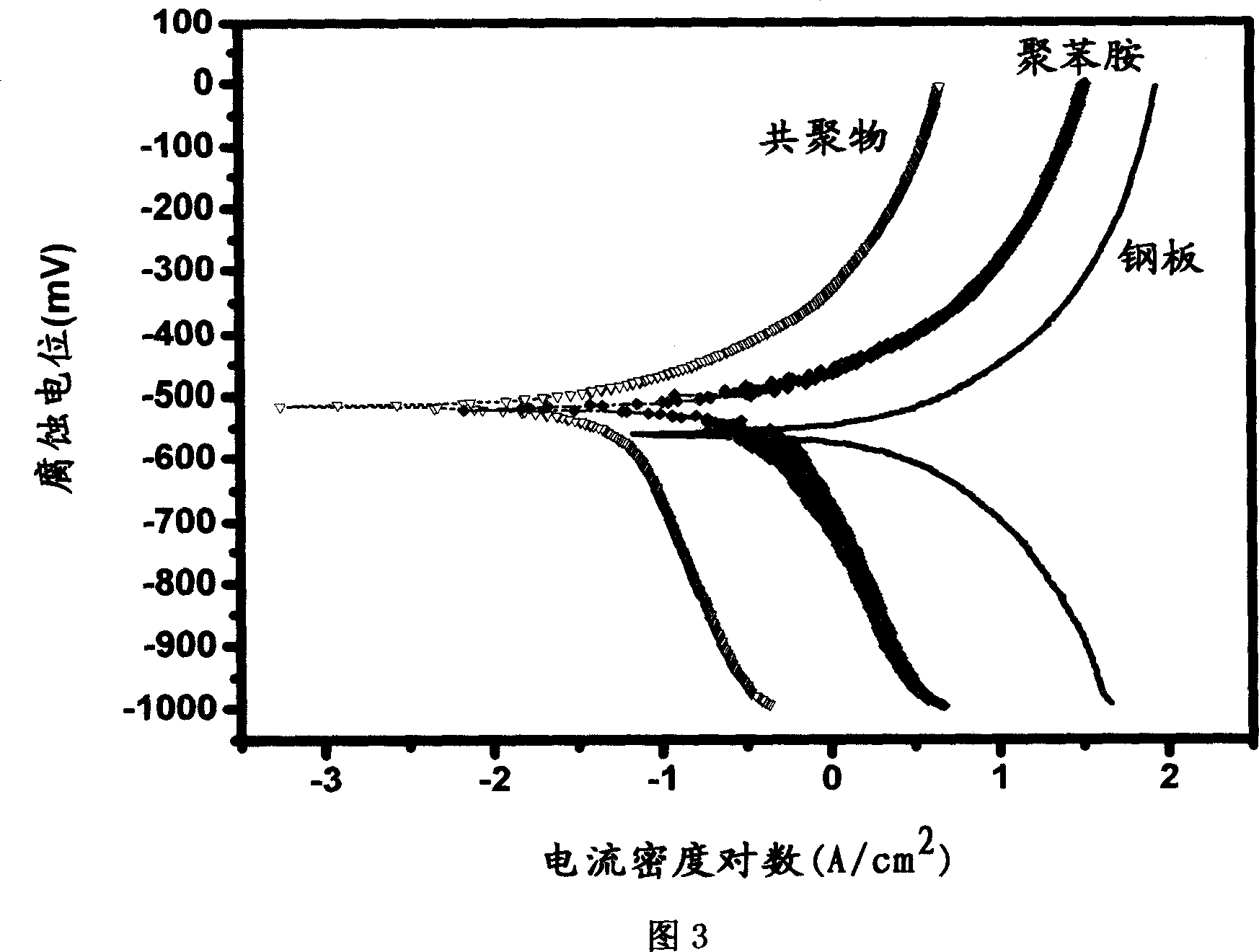
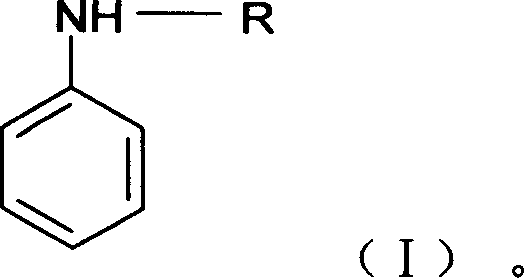
Anticorrosion paint containing aniline copolymer and its preparation and use
An aniline copolymer and anti-corrosion coating technology, applied in anti-corrosion coatings, epoxy resin coatings, coatings and other directions, can solve the problems of inability to achieve industrialization, inability to form large-area films, etc., and achieve a wide range of applications, improved solubility, The effect of good anti-corrosion performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of the anticorrosion coating of N-ethylaniline / aniline copolymer, its step is:
[0035] (1) Synthesis of N-ethylaniline / aniline copolymer
[0036] Dissolve aniline and N-ethylaniline in 80mL reaction medium (such as 1.0mol / L hydrochloric acid solution) in a certain proportion, stir with a magnetic stirrer for 30 minutes to make it evenly dispersed, and then put it into the temperature in a water bath. Add 20 mL of an oxidizing agent solution (such as a hydrochloric acid solution of ammonium persulfate) dropwise into the monomer solution at a rate of 3 seconds, and stir while adding. Reacted at a constant temperature for 24h to obtain a dark green polymer. Subsequently, the reaction solution was filtered, washed with distilled water until the filtrate was colorless, and dried to obtain a doped polymer sample. For dedoped polymer samples, 0.2mol / L alkaline water (ammonia solution or NaOH solution) can be used for dedoping treatment, magnetically ...
Embodiment 1
[0050] Add 5.840mL of N-ethylaniline and 2.061mL of aniline (the molar ratio of the monomers is 20:80) into 80mL of 1mol / L hydrochloric acid reaction medium, place the reaction system in a water bath at 0-5°C, and pass 18.256g of the oxidizing agent over Dissolve ammonium sulfate in 20mL of 1.0mol / L hydrochloric acid aqueous solution, add this solution dropwise to the monomer solution, continue to react for 24 hours, filter, wash with distilled water until the filtrate is colorless, and dry to obtain doped N- Ethylaniline / aniline copolymer. The yield was 68.8%, and its conductivity was 3.36×10 -2 S / cm.
[0051] Move the doped N-ethylaniline / aniline copolymer obtained in Example 1 into a beaker, add ammonia water and magnetically stir for 24 hours, filter, then wash with a large amount of distilled water until neutral, filter, and dry to obtain the dedoped state N-ethylaniline / aniline copolymer with a conductivity of 1.95×10 -9 S / cm.
Embodiment 2~3
[0053] The preparation process of the copolymer is the same as in Example 1, changing the addition amount of the two monomers, adding 1.030mL N-ethylaniline and 6.57mL aniline (monomer molar ratio is 10:90) and 5.15mL N-ethylaniline respectively With 3.65mL aniline (monomer molar ratio is 50: 50), the yield of doping state N-ethylaniline / aniline copolymer is respectively 74.3% and 45.6%, and their electrical conductivities are respectively 9.64×10 -2 S / cm, 7.46×10 -7 S / cm. The corresponding conductivity of the dedoped N-ethylaniline / aniline copolymer is 5.05×10 -9 S / cm and 4.12×10 -10 S / cm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com