Preparation method of benzoxazine intermediate containing active function groups
A technology of active functional group and benzoxazine, applied in the field of thermosetting resin and its preparation, can solve the problems of low thermal performance, invisible, poor toughness, etc., and achieve the effect of lowering curing temperature, reducing dosage, and high heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
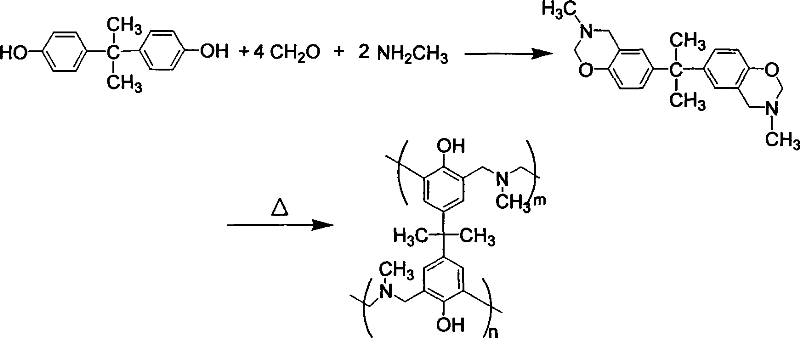
[0034]Example 1 Synthesis of 3-hexylamino-6-p-hydroxycumene-3,4-dihydro-2H-1,3-benzoxazine
[0035] Add 75ml of chloroform, 0.7g of triethylamine and 5.15g of paraformaldehyde in sequence into a 250ml three-necked flask equipped with a stirrer and a condenser, and stir to mix. Slowly add 9.3g of hexamethylenediamine, control the reaction temperature not to exceed 30°C, add 18.3g of bisphenol A after 15 minutes of reaction, raise the temperature to reflux, and stop the reaction after the mixed solution reacts at reflux temperature for 5 hours to obtain a light yellow oil in the lower layer phase, a phase-separation system of the upper transparent aqueous phase. The reaction product was poured into excess methanol for precipitation, and filtered with 1N NaHCO 3 The aqueous solution was washed three times, distilled under reduced pressure and dried in vacuo to obtain a white powder namely 3-hexylamino-6-p-hydroxycumene-3,4-dihydro-2H-1,3-benzoxazine, the yield 85%.
Embodiment 2
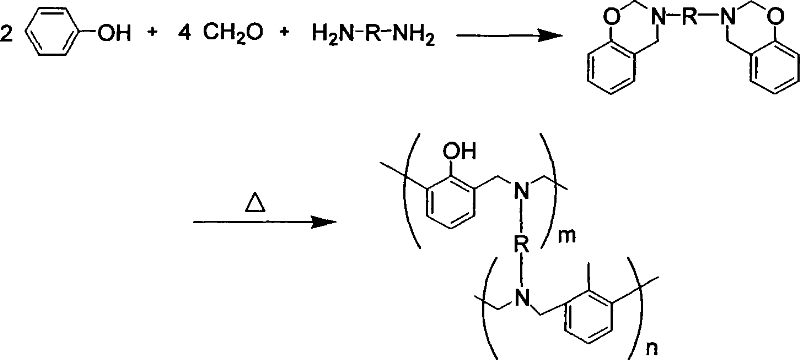
[0036] Example 2 Synthesis of 3-ethylamino-6-p-hydroxybenzophenone-3,4-dihydro-2H-1,3-benzoxazine
[0037] Add 75ml of chloroform, 0.7g of triethylamine and 24ml of formaldehyde solution in sequence to a 250ml three-neck flask equipped with a stirrer and a condenser, and stir to mix. Slowly add 4.5g of ethylenediamine, control the reaction temperature not to exceed 30°C, add 16.1g of 4,4'-p-hydroxybenzophenone after 15 minutes of reaction, raise the temperature to reflux, react the mixed solution at the reflux temperature for 5 hours, and then stop the reaction . The reaction mixture was filtered and washed with 1N NaHCO 3 The aqueous solution was washed three times, distilled under reduced pressure and dried under vacuum to obtain a white powder, namely 3-ethylamino-6-p-hydroxybenzophenone-3,4-dihydro-2H-1,3-benzoxazine, with a yield of 80 %.
Embodiment 3
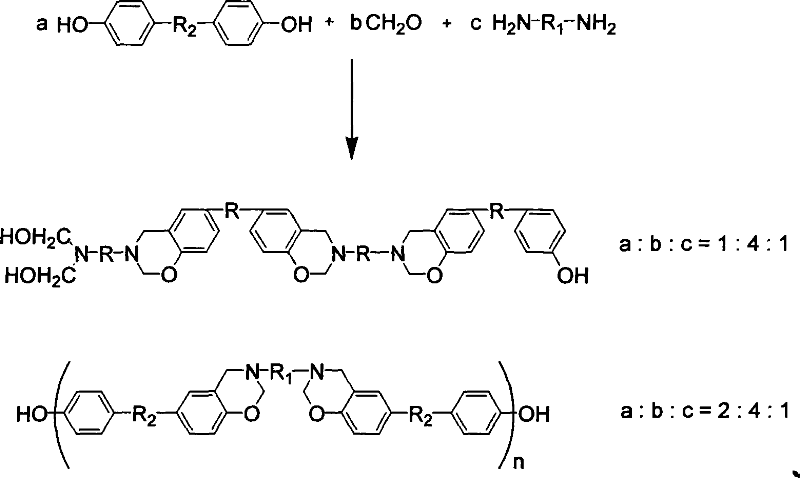
[0038] Example 3 Synthesis of 3-p-aminodiphenylmethane-6-p-hydroxycumene-3,4-dihydro-2H-1,3-benzoxazine
[0039] Add 75ml of chloroform, 0.7g of triethylamine and 24ml of formaldehyde solution in sequence to a 250ml three-neck flask equipped with a stirrer and a condenser, and stir to mix. Slowly add 14.9g of diphenylmethanediamine, control the reaction temperature not to exceed 30°C, add 17.1g of bisphenol A after 15 minutes of reaction, raise the temperature to reflux, react the mixed solution at reflux temperature for 5 hours, stop the reaction, and obtain a light yellow lower layer A phase-separation system with an oil phase and an upper transparent water phase. The reaction product was poured into methanol for precipitation, and filtered with 1N NaHCO 3 After washing with aqueous solution, distilling under reduced pressure and drying in vacuo, a white powder was obtained, namely 3-p-aminodiphenylmethane-6-p-hydroxyisopropylbenzene-3,4-dihydro-2H-1,3-benzoxazine, Yield 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com