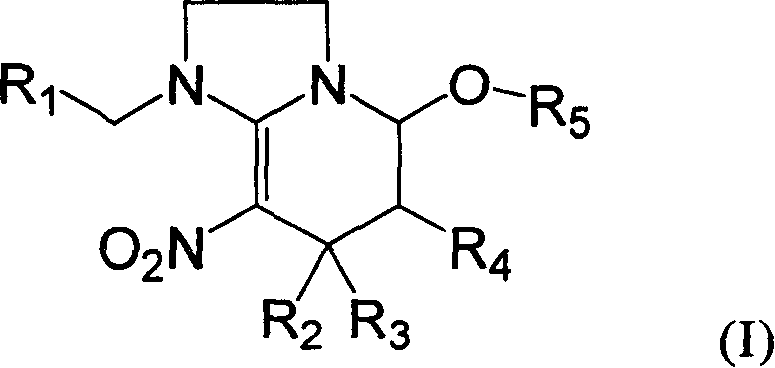
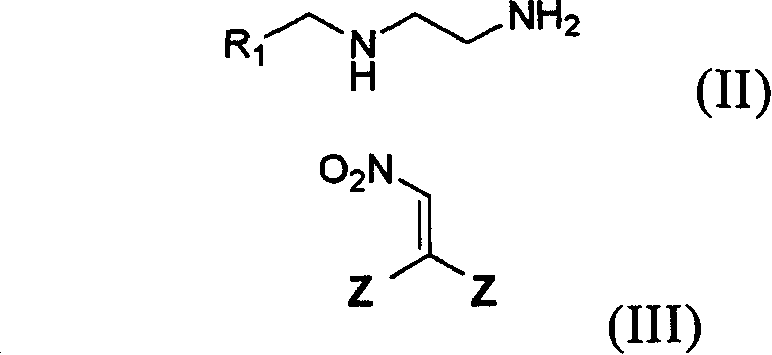
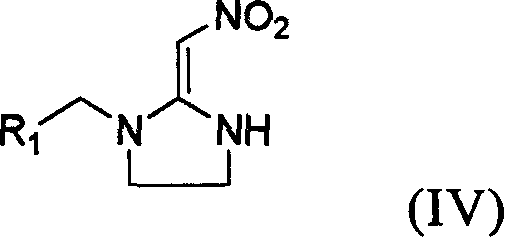
Preparation method and use of compound with high insecticidal activity
A compound and plant technology, applied in the field of nitromethylene derivatives, can solve the problems of complex preparation process, high cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] 1-(6-Chloro-3-methylpyridinyl)-7-methyl-8-nitro-1,2,3,5,6,7-hexahydroimidazo[1,2-a]pyridine- Synthesis of 5-alcohol (Compound 1):
[0069] (1) Synthesis of 2-nitro-ethylene-1,1-disulfide potassium
[0070]
[0071] Put 4.0g (0.03mol) of nitromethane and 6ml (0.05mol) of carbon disulfide in a 100ml three-necked flask, add 10ml of ethanol as a solvent, and start stirring. Weigh 8g (0.14mol) of potassium hydroxide and dissolve it with 40ml of ethanol, and slowly add it dropwise to the above solution at room temperature (about 30min). Because the reaction process is exothermic, the dropping rate depends on the reaction temperature at the time, and the temperature should be controlled between 30 and 35°C. After the addition is complete, let the reaction continue to stir for 2 hours. Finally, the solid was filtered out, and the crude product was obtained as a brown-yellow powdery solid with a yield of 72%.
[0072] (2) Synthesis of 1,1-Dithiomethyl-2-nitroethylene
[0073]
...
Embodiment 2
[0089] 1-(6-Chloro-3-methylpyridyl)-5-methoxy-7-methyl-8nitro-1,2,3,5,6,7-hexahydroimidazole [1,2- ] Synthesis of Pyridine (Compound 2)
[0090] (1) Synthesis of 1,1-dichloro-2-nitroethylene
[0091]
[0092] In a three-necked flask equipped with stirring, a mixture of 20.85 g (0.2055 mol) of 36% hydrochloric acid and 19.9 g (0.2055 mol) of 65% nitric acid was added. 15.5g (0.1575mol) of vinylidene chloride was added dropwise, the temperature of the dropwise addition was controlled between 20-25°C, and the temperature was kept and stirred for 3 hours. Add 50ml of dichloromethane to extract, wash with water, and control the pH of the oil layer to about 3-4. Add 5-6 grams of alkali to 120ml of water to dissolve, cool to 0°C, gradually add to the oil layer, and keep the temperature at 0°C. After the addition is complete, stir vigorously for another 5 minutes. Extraction with dichloromethane, separate layers, wash the oil layer with water, dry and filter to obtain about 13.5-17.5 g ...
Embodiment 3
[0107] 1-(6-Chloro-3-methylpyridinyl)-5-ethoxy-7-methyl-8nitro-1,2,3,5,6,7-hexahydroimidazole [1,2- ] The synthesis of pyridine (compound 3), the reaction equation is as follows:
[0108]
[0109] Combine 10.16g (0.04mol) of 2-chloro-5-(2-nitromethylene-imidazolidine-1-ylmethyl)-pyridine, 100ml of absolute ethanol, about 5ml of crotonaldehyde, catalytic amount of AcOH Placed in a 250ml round-bottomed flask, refluxed, TLC followed the reaction, after the reaction, the solvent was removed, column chromatography was separated to obtain a pure yellow powder, and the pure product was separated by column chromatography as a yellow powder solid with a yield of 75 %, mp = 138.8-140.3°C. 1 H NMR(500MHz, CDCl 3 ): 8.31(d, J=2Hz, 1H, pyridine-H), 7.87(dd, J 1 = 2Hz, J 2 = 8Hz, 1H, pyridine-H), 7.33 (d, J=8Hz, 1H, pyridine-H), 4.76 (dd, J 1 =15Hz, J 2 =15Hz, 2H, -CH 2 -N-), 4.56(t, J 1 =3Hz, J 2 = 3Hz, 1H, -CHO-), 3.60 (m, 4H, imidazolidine-H), 3.57 (m, 2H, -O-CH 2 -), 3.53(m, 1H, -CHCH 2 -...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com