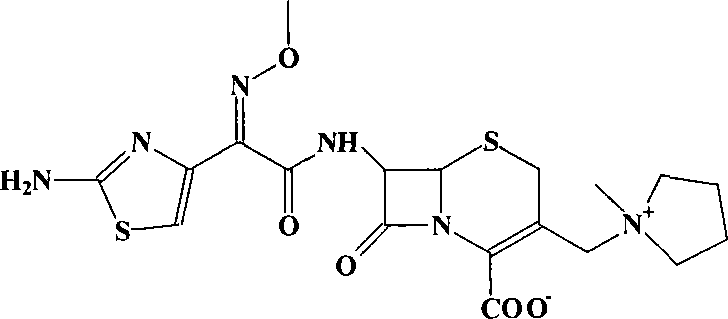
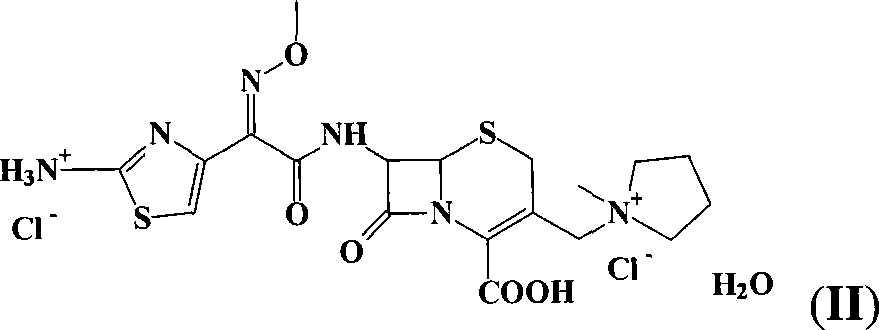
Preparation of cefepime hydrochlorice by sodium salt precipitation method
A technology of cefepime hydrochloride and sodium salt, which is applied in the field of preparation of cephalosporin compounds, can solve problems such as increased impurities, deepened color of feed liquid, and influence on product purity, so as to reduce pollution, reduce dosage, and increase production rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Dissolve 171g of sodium isooctanoate (1.03mol) in 900mL of water and 200mL of acetone at room temperature (15-25°C), and add 300g of cefepime sulfate (0.52mol, weight based on anhydrous substance) in batches under effective stirring to form Good fluidity paste system. After 10 minutes, add 1600 mL of acetone and appropriate amount of activated carbon, and stir for 30 minutes. The solid was isolated by centrifugation or suction filtration. Wash the filter cake with 500 mL of acetone-water 2:1 (v / v) mixed solution, and combine the filtrates. 18% HCl was added to the filtrate with stirring to pH 1.0-1.5. Then 8 L of acetone was added over 1 hour. The temperature of the system was lowered to 5-10° C., and the stirring was continued for 1 hour. Suction. The filter cake was washed with acetone and dried under vacuum at 40° C. to obtain about 270 g of white crystalline powder. The purity is 99.7%, the water content is 3.8% (K-F method), and the residual acetone is 0.25%....
Embodiment 2
[0033] Add 300g of cefepime sulfate (0.52mol, the weight is based on anhydrous matter) to 900mL of water at room temperature (15-25°C), and add about 20% sodium hydroxide aqueous solution dropwise under vigorous stirring until the pH of the system is 5.5-6.5 between. Then add 2100mL acetone and an appropriate amount of activated carbon, and stir for 30 minutes. The solid was isolated by centrifugation or suction filtration. Wash the filter cake with 500 mL of acetone-water 2:1 (v / v) mixed solution, and combine the filtrates. 30% HCl was added to the filtrate with stirring until the pH was 1.0-1.5. Then 8 L of acetone was added over 1 hour. The temperature of the system was lowered to 5-10° C., and the stirring was continued for 1 hour. Suction. The filter cake was washed with acetone and dried under vacuum at 40° C. to obtain about 270 g of white crystalline powder. The purity is above 99.7%, the water content is 3-4%, and the residual acetone is less than 0.4%. The pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com