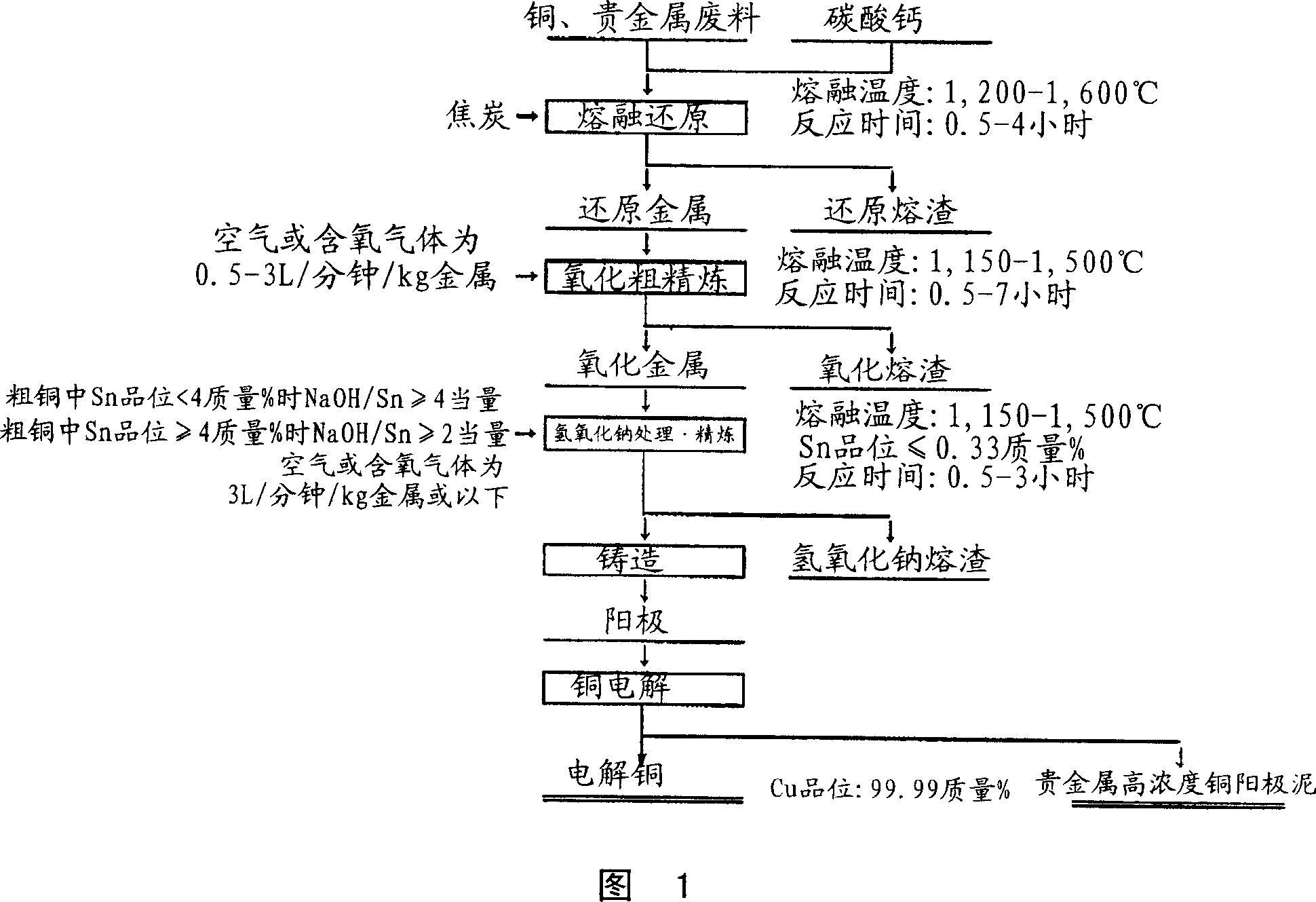
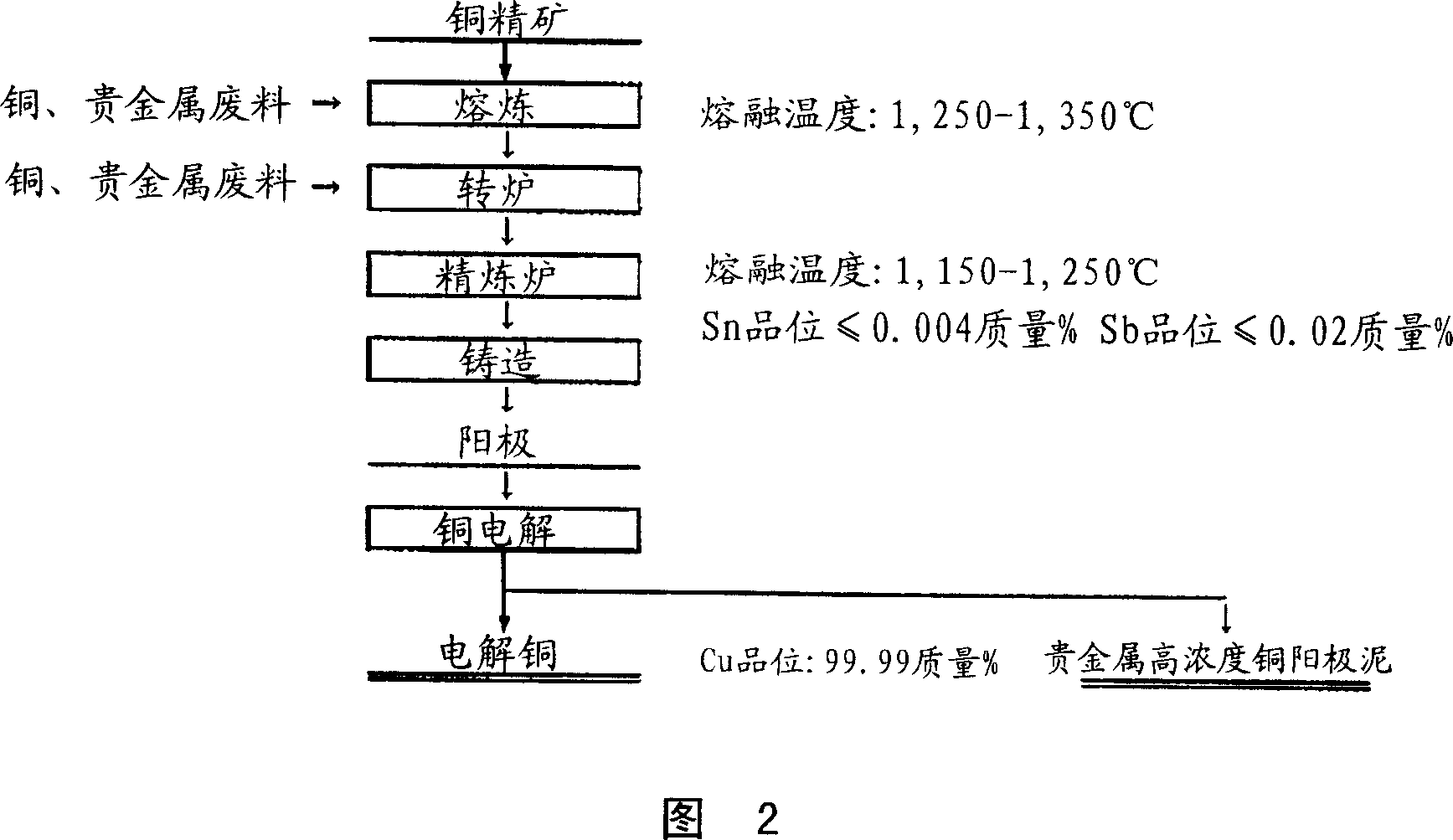
Dry type refining method for copper
A dry, electrolytic refining technology, applied in the direction of improving process efficiency, can solve undisclosed problems, and achieve the effects of improving productivity, reducing transfer rate, and reducing circulation processing capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment -1
[0029] Copper obtained by incinerating copper containing at least one precious metal of gold, silver, platinum, palladium, rhodium, ruthenium, and tin, and flammable electronic component materials, power supply substrates, etc. 60kg of precious metal scrap raw materials, 140kg of electroplating waste residues such as electronic component materials, 20kg of precious metal-containing waste liquid treatment residues produced in the wet smelting process of precious metals, 26kg of calcium carbonate as a flux, 15kg of coke as a reducing agent for a smelting reduction furnace, The smelting reduction treatment was carried out at 1,300-1,400° C. for 3 hours, and then 146 kg of reduced metal and 68 kg of reduced slag were separated. The reduced metal was melted in the oxidation furnace at a temperature range of 1,200°C-1,250°C, and then air was blown in at a flow rate of 0.8 L / min / kg metal for 2 hours from the blowing port, and then the oxidized crude was collected from the oxidation fu...
Embodiment -2
[0034] In order to make the tin grade of the crude oxidized metal prepared in Example-1 2% by mass, the crude oxidized metal and tin metal as a reagent were put into a graphite crucible and melted at 1,200°C for 1 hour using an externally heated electric furnace. . Then, the graphite crucible was taken out from the externally heated electric furnace and cooled to obtain a metal whose tin grade was adjusted to 2% by mass.
[0035] Except having used the metal whose tin level adjusted to 2 mass % prepared above, it processed and analyzed similarly to Example 1. The results are shown in Table 2.
[0036]
[0037] As can be seen from the results in Table 2, for the tin removal treatment in Example-2, as long as 4.5 equivalents or more of sodium hydroxide is added to the theoretical amount of tin, an anode grade that can be electrolyzed in the electrolytic refining step of copper can be obtained.
Embodiment -3
[0039] In order to make the tin grade of the crude oxidized metal prepared in Example-1 4% by mass, the crude oxidized metal and tin metal as a reagent were put into a graphite crucible and melted at 1,200°C for 1 hour using an externally heated electric furnace. . Then, the graphite crucible was taken out from the externally heated electric furnace and cooled to obtain a metal whose tin grade was adjusted to 4% by mass.
[0040] The same treatment and analysis as in Example-1 were performed except that the metal prepared above with the tin grade adjusted to 4% by mass was used. The results are shown in Table 3.
[0041] Table 3 shows the results of analysis in the same manner as in Example-1, except that the copper metal having a tin grade of 4% by mass prepared above was used.
[0042]
[0043] As can be seen from the results in Table 3, for the tin removal treatment in Example 3, as long as 2 equivalents or more of sodium hydroxide is added relative to the theor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com