Process for alkyl aryl sulfide derivatives and new sulfide compounds
A technology for alkyl aryl and aryl halides, applied in the field of alkyl aryl sulfide derivatives and new sulfides, can solve the problems of troublesome methods, easy formation of disulfides, difficult to handle and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
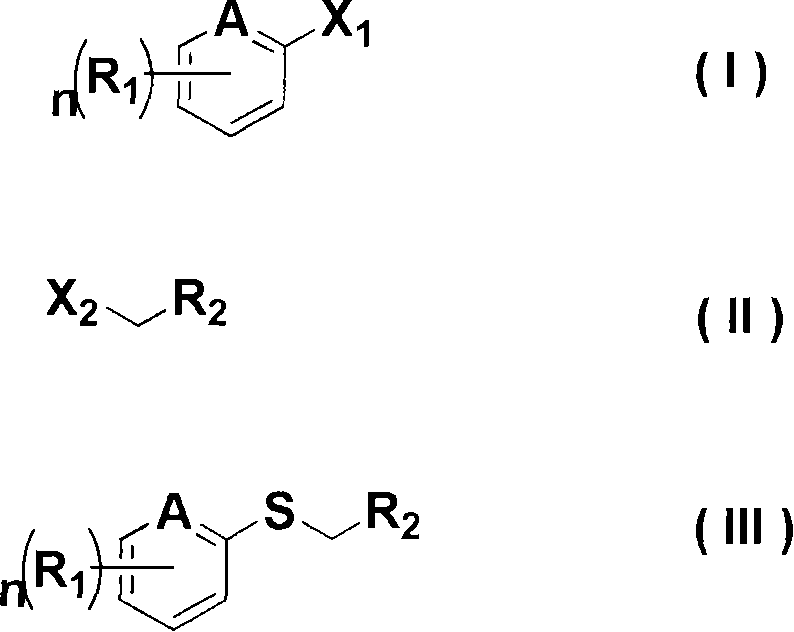
[0040] In the production method according to the present invention, the compound of the chemical formula (I) used as a raw material is widely known in the art and can be obtained commercially.
[0041] The preparation method according to the present invention will be described in detail below.
[0042] [Method A] Production of an alkylaryl sulfide represented by the chemical formula (III) having an electron-donating or electron-withdrawing substituent
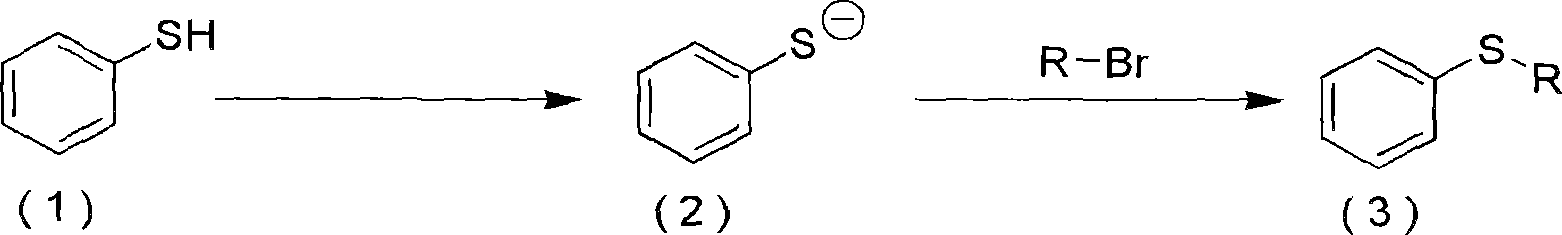
[0043] The alkylaryl sulfide represented by the chemical formula (III) is obtained by reacting the compound represented by the chemical formula (I) with an alkyllithium organometallic reagent and sulfur, and then reacting with the compound represented by the chemical formula (II).
[0044] A dry solvent such as diethyl ether, tetrahydrofuran, hexane, and heptane can be used alone or a mixture of two or more of them can be used in this method. Among them, diethyl ether, tetrahydrofuran, and a mixture of diethyl ether and tetrah...
Embodiment 1
[0058] [Example 1] The preparation of benzyl 2-trifluoromethylphenyl sulfide
[0059]
[0060] Under a nitrogen atmosphere, 271 µl of 1-bromo-2-(trifluoromethyl)-benzene was completely dissolved in 15 ml of dry tetrahydrofuran, and the mixture was cooled to -78°C. To this mixture was slowly added 1.25 ml of n-butyllithium (1.6M in cyclohexane, 1.0 eq.) over 1 minute. After stirring for another 10 minutes, 64 mg (2 mmol, 1.0 equivalent) of sulfur powder was added at the same temperature at one time. The mixture was further stirred at the same temperature for 10 minutes to completely dissolve sulfur, and then 236 µl (2 mmol, 1.0 equivalent) of benzyl bromide was slowly added thereto. The reaction was carried out allowing the overall temperature of the reaction mixture to rise to room temperature within 20 minutes. The reaction was monitored by TLC, and at the end of the reaction, 15 ml of ammonium chloride aqueous solution was added thereto to terminate the reaction. The o...
Embodiment 2
[0063] [embodiment 2] the preparation of benzyl 3-trifluoromethyl phenyl sulfide
[0064]
[0065] The same procedure as described in Example 1 was repeated except that 276 µl (2 mmol) of 1-bromo-3-(trifluoromethyl)-benzene was used instead of 1-bromo-2-(trifluoromethyl)-benzene. After purification, 381 mg of the title compound was obtained (yield: 71%).
[0066] 1 H-NMR (300MHz, CDCl 3 )δ: 7.51 (br s, 1H), 7.39 (t, 2H), 7.33 (d, 1H), 7.28 (m, 5H), 4.13 (s, 2H).
[0067] 13C-NMR (75.5MHz, CDCl 3 )δ: 138.3, 137.0, 133.0, 131.6 (q, J=32Hz), 129.5, 129.2, 129.0, 128.9, 127.9, 126.5 (q, J=3.7Hz), 123.3, 39.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com