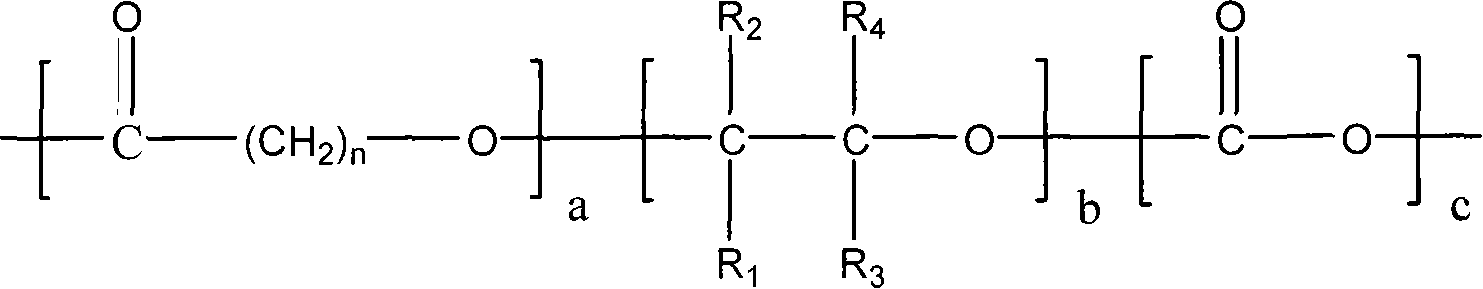
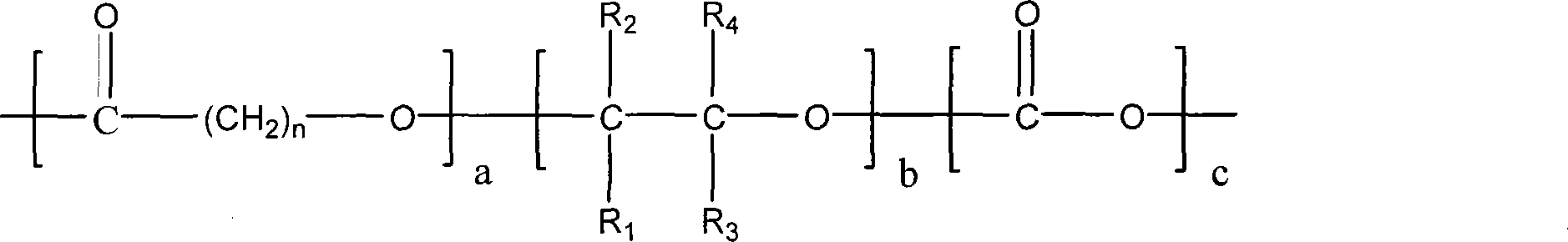
Terpolymer for controllable degradation of carbon dioxide/epoxide/lactone and preparation method thereof
A technology of terpolymer and epoxy, which is applied in the field of polymer chemistry, can solve problems such as unsatisfactory degradation rate, and achieve the effects of improving physical and mechanical properties, increasing molecular weight, and improving degradation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Preparation of the terpolymer (PPCG) of carbon dioxide / propylene oxide / γ-butyrolactone: the polymerization reaction was carried out in a 300mL magnetic autoclave. Propylene oxide 50mL, benzene 50mL, γ-butyrolactone and propylene oxide are in a certain ratio, respectively 0:5, 1:5, 2:5, 3:5, 4:5, 5:5, fully stirred for 0.5h , then pass into the CO 2 The pressure of the gas to the reaction system is 3.5-4Mpa. Heated, polymerized at 60°C for 24h, stopped heating and stirring, cooled to room temperature, and discharged the remaining gas. After discharging, the copolymer was washed with 5% hydrochloric acid ethanol, washed three times with ethanol, and dried to obtain a crude product. Dissolve in dichloromethane, centrifuge to remove insoluble matter and residual catalyst, evaporate to remove solvent, and vacuum dry at 35°C until constant weight. The polymers PPC, PPCG1, PPCG2, PPCG3, PPCG4, PPCG5 were obtained respectively. Their viscosity-average molecular weights are:...
Embodiment 2
[0018] Preparation of the terpolymer (PPCCL) of carbon dioxide / propylene oxide / ε-caprolactone: the polymerization reaction was carried out in a 300mL magnetic autoclave. Propylene oxide 50mL, toluene 60mL, ε-caprolactone and propylene oxide are in a certain ratio, respectively 1:3, 2:3, 3:3, after fully stirring for 0.5h, then introduce CO 2 The pressure of the gas to the reaction system is 3.5-4Mpa. Heated, polymerized at 60°C for 24h, stopped heating and stirring, cooled to room temperature, and discharged the remaining gas. After discharging, the copolymer was washed with 5% hydrochloric acid ethanol, washed three times with ethanol, and dried to obtain a crude product. Dissolve in dichloromethane, centrifuge to remove insoluble matter and residual catalyst, evaporate to remove solvent, and vacuum dry at 35°C until constant weight. The polymers PPCCL1, PPCCL2 and PPCCL3 were obtained respectively. Their viscosity-average molecular weights are: 51200, 67100, 57300 respect...
Embodiment 3
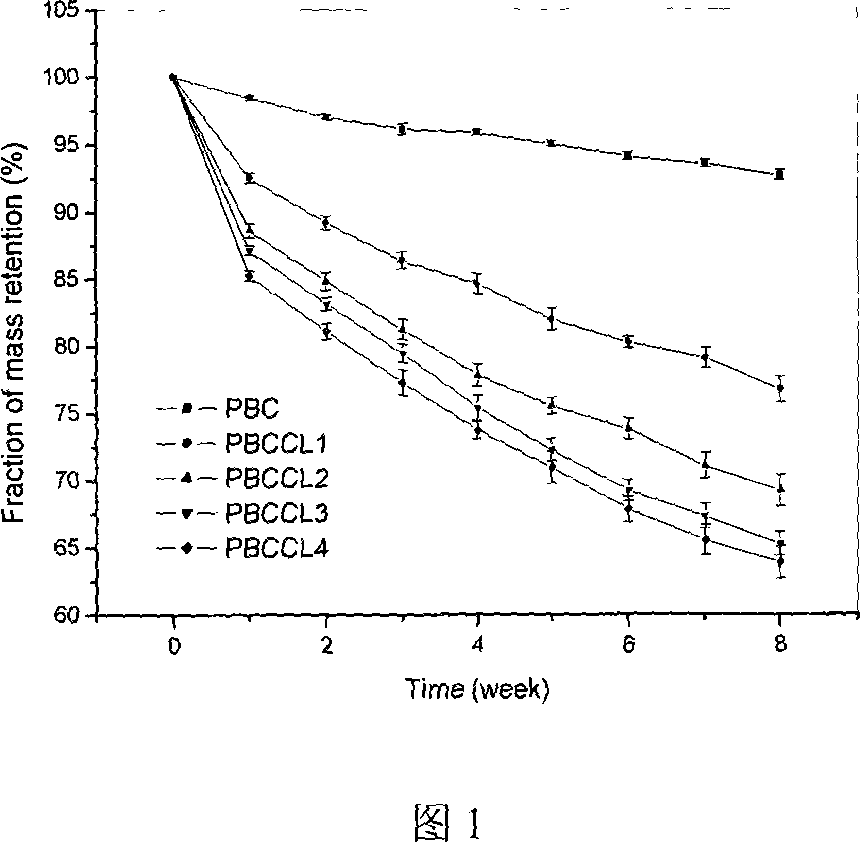
[0020] Preparation of terpolymer (PBCCL) of carbon dioxide / 1,2 epoxybutylene / ε-caprolactone: The polymerization reaction was carried out in a 300mL magnetic autoclave, and after purging the autoclave with nitrogen for 10min, the catalyst was quickly added 1.00g, 32mL of 1,2-butylene oxide, 50mL of toluene, ε-caprolactone and 1,2-butylene oxide in a certain ratio, respectively 0:4, 1:4, 2:4, 3:4, 4:4, after fully stirring for 0.5h, then introduce CO 2 The pressure of the gas to the reaction system is 3.5-4Mpa. Heated, polymerized at 70°C for 36h, stopped heating and stirring, cooled to room temperature, and discharged the remaining gas. After discharging, the copolymer was washed with 5% hydrochloric acid ethanol, washed three times with ethanol, and dried to obtain a crude product. Dissolve in dichloromethane, centrifuge to remove insoluble matter and residual catalyst, evaporate to remove solvent, and vacuum dry at 35°C until constant weight. The polymers PBC, PBCCL1, PBCC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com