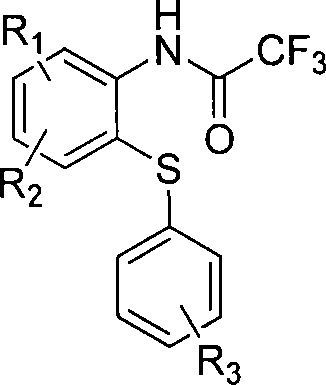
2,2,2-trifluoro-n-(2-arylsulf)-a rylamide derivatives and preparing method
A technology of aryl acetamide and derivatives, applied in 2 fields, can solve the problems of metal palladium catalyst air instability, high ligand toxicity, high price, etc., and achieve the effects of easy post-processing, small environmental impact, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
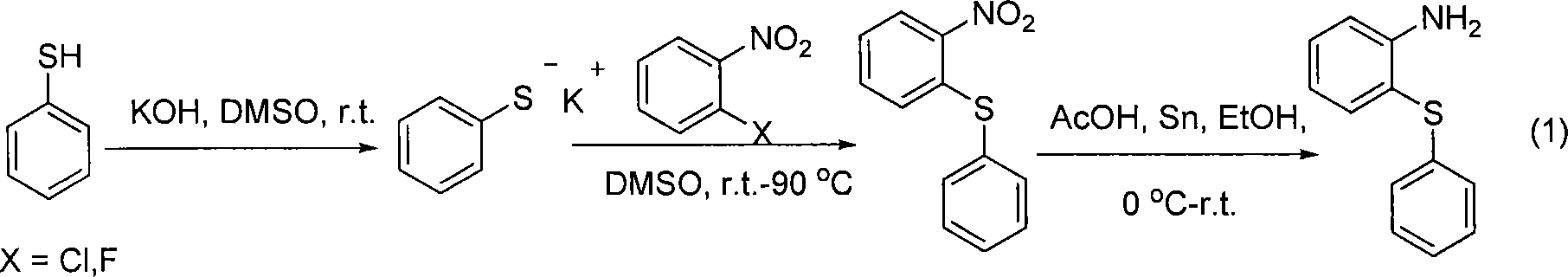
[0033] Example 1: 2,2,2-trifluoro-N-(2-p-methylphenylthiophenyl)acetamide. Add 2ml 1,2-dimethoxyethane, 0.5mmol 2,2,2-trifluoro-N-(2-iodophenyl)acetamide, 1.1 equivalent p-methylthiophenol to the sealed tube , 2.0 equivalents of potassium carbonate, 0.05mmol cuprous iodide, 0.1mmol L-proline, under nitrogen protection, stirred at 60°C for 40h. Add 10 ml of water and 20 ml of ethyl acetate to the reaction system cooled to room temperature, stir, filter the insoluble matter, and extract the water layer with 10 ml of ethyl acetate each time until thin layer chromatography shows that there is no product in the water layer. The organic layers were combined, and most of the organic solvent was removed by distillation under reduced pressure, and the residue was separated by silica gel column chromatography (petroleum ether: ethyl acetate=40:1) to obtain the product 2,2,2-trifluoro-N-(2 - p-methylphenylthiophenyl)acetamide.
[0034] The structure is:
[0035]
[0036] Molecular ...
Embodiment 2
[0045]Example 2: 2,2,2-trifluoro N-(4-nitro-2-p-tolylthiophenyl)acetamide, add 3ml of 1,2-dimethoxyethane into the sealed tube, 1.0mmol 2,2,2-trifluoro N-(4-nitro-2-iodophenyl)acetamide, 2.0 equivalents of p-methylthiophenol, 2.5 equivalents of potassium carbonate, 0.10mmol cuprous iodide, 0.20 mmol L-proline, under nitrogen protection, stirred at 60°C for 40h. Add 5 ml of water and 10 ml of ethyl acetate to the reaction system cooled to room temperature, stir, filter the insoluble matter, and extract the water layer with 15 ml of ethyl acetate each time until thin layer chromatography shows no product in the water layer. The organic layers were combined, and most of the organic solvent was removed by distillation under reduced pressure, and the residue was separated by silica gel column chromatography (petroleum ether: ethyl acetate=40:1) to obtain the product 2,2,2-trifluoro-N-(4 -nitro-2-p-tolylthiophenyl)acetamide.
[0046] The structure is:
[0047]
[0048] Molecul...
Embodiment 3
[0057] Example 3: 2,2,2-trifluoro-N-(2-phenylthiophenyl)acetamide, add 2.5ml 1,2-dimethoxyethane into the sealed tube, 0.5mmol 2 , 2,2-trifluoro-N-(2-iodophenyl)acetamide, 1.5 equivalents of thiophenol, 2.5 equivalents of potassium carbonate, 0.05 mmol cuprous iodide, 0.1 mmol L-proline, under nitrogen protection, Stir at 60°C for 40h. Add 10 ml of water and 20 ml of ethyl acetate to the reaction system cooled to room temperature, stir, filter the insoluble matter, and extract the water layer with 10 ml of ethyl acetate each time until thin layer chromatography shows that there is no product in the water layer. The organic layers were combined, and most of the organic solvent was removed by distillation under reduced pressure, and the residue was separated by silica gel column chromatography (petroleum ether: ethyl acetate=80:1) to obtain the product 2,2,2-trifluoro-N-(2 -phenylthiophenyl)acetamide.
[0058] The structure is:
[0059]
[0060] Molecular formula: C 14 h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com