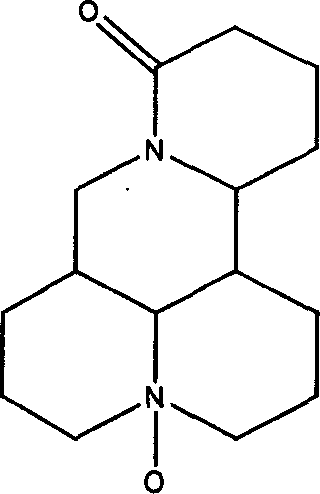
Medicinal composition of oxymatrine and polysaccharide
A composition, the technology of matrine, applied in the field of medicine, to achieve the effect of ensuring safety, small quality difference and definite curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Example 1 Preparation of Ganoderma lucidum polysaccharide
[0124] Take Ganoderma lucidum medicinal materials, crush them into coarse particles, add 4 times the amount of water to wet overnight, decoct three times the next day, add 12 times the amount of water for 3 hours for the first time, add 10 times the amount of water for 2 hours for the second time, and decoct for 2 hours for the second time. Add 10 times the amount of water three times and cook for 1 hour, combine the decoction, filter, concentrate the filtrate to a relative density of 1.10-1.15, filter, remove the protein from the filtrate by the Sevage method, filter, add ethanol to the filtrate until the alcohol content is 60%, stir well, refrigerate for 24 hours, filter, collect the filter cake, add an appropriate amount of water and stir to dissolve, filter, add ethanol to the filtrate until the alcohol content is 85%, stir well, refrigerate for 24 hours, filter Then, collect the filter cake, add appropri...
Embodiment 2
[0135] Preparation of 2 KLX composition powder for injection
[0136] 1. Prescription:
[0137] Matrine 400g
[0138] Ganoderma lucidum polysaccharide 28.8g (equivalent to 3kg of the original medicinal material)
[0139] Mushroom polysaccharide 8g
[0140] Polysorbate 80 50g
[0141] Mannitol 300g
[0142] Sterile water for injection added to 5000ml
[0143]
[0144] A total of 1000 pieces were prepared
[0145] 2. Specific steps:
[0146] 1) First, aseptically treat the container for liquid dispensing, antibiotic glass bottle, rubber stopper, etc.
[0147] 2) Weigh the raw materials and auxiliary materials according to the prescription amount.
[0148] 3) Add an appropriate amount of water for injection to matrine and mannitol, heat and stir to dissolve, take 70% of sterile water for injection, add polysorbate 80, heat and stir to dissolve, and then add the prescription amount of Ganoderma lucidum polysaccharide and shii...
Embodiment 3
[0156] Example 3 Preparation of KLX composition water injection
[0157] 1. Prescription:
[0158] Matrine 400g
[0159] Ganoderma lucidum polysaccharide 28.8g (equivalent to 3kg of the original medicinal material)
[0160] Mushroom polysaccharide 8g
[0161] Polysorbate 80 50g
[0162] Add water for injection to 5000ml
[0163]
[0164] A total of 1000 pieces were prepared
[0165] 2. Specific steps:
[0166] 1) Dispose of the pipelines and containers for liquid dispensing one day in advance, and rinse with fresh water for injection before use.
[0167] 2) Add an appropriate amount of water for injection to matrine, heat and stir to dissolve, take 70% sterile water for injection, add polysorbate 80, heat and stir to dissolve, then add the prescription amount of Ganoderma lucidum polysaccharide and lentinan, and stir Completely dissolved. Combine the above solutions and add sterile water for injection to 5000ml.
[0168] 3) Add 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com