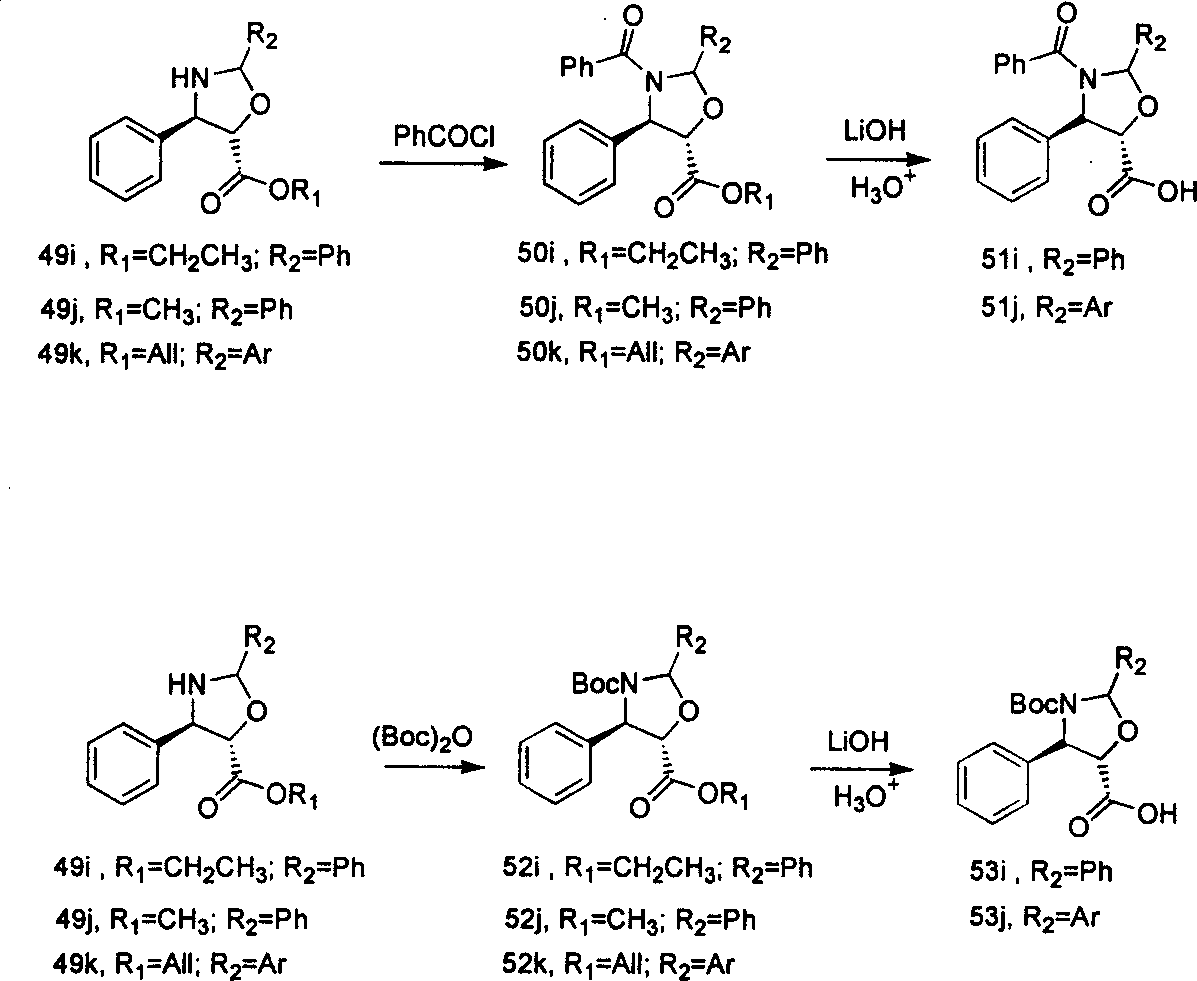
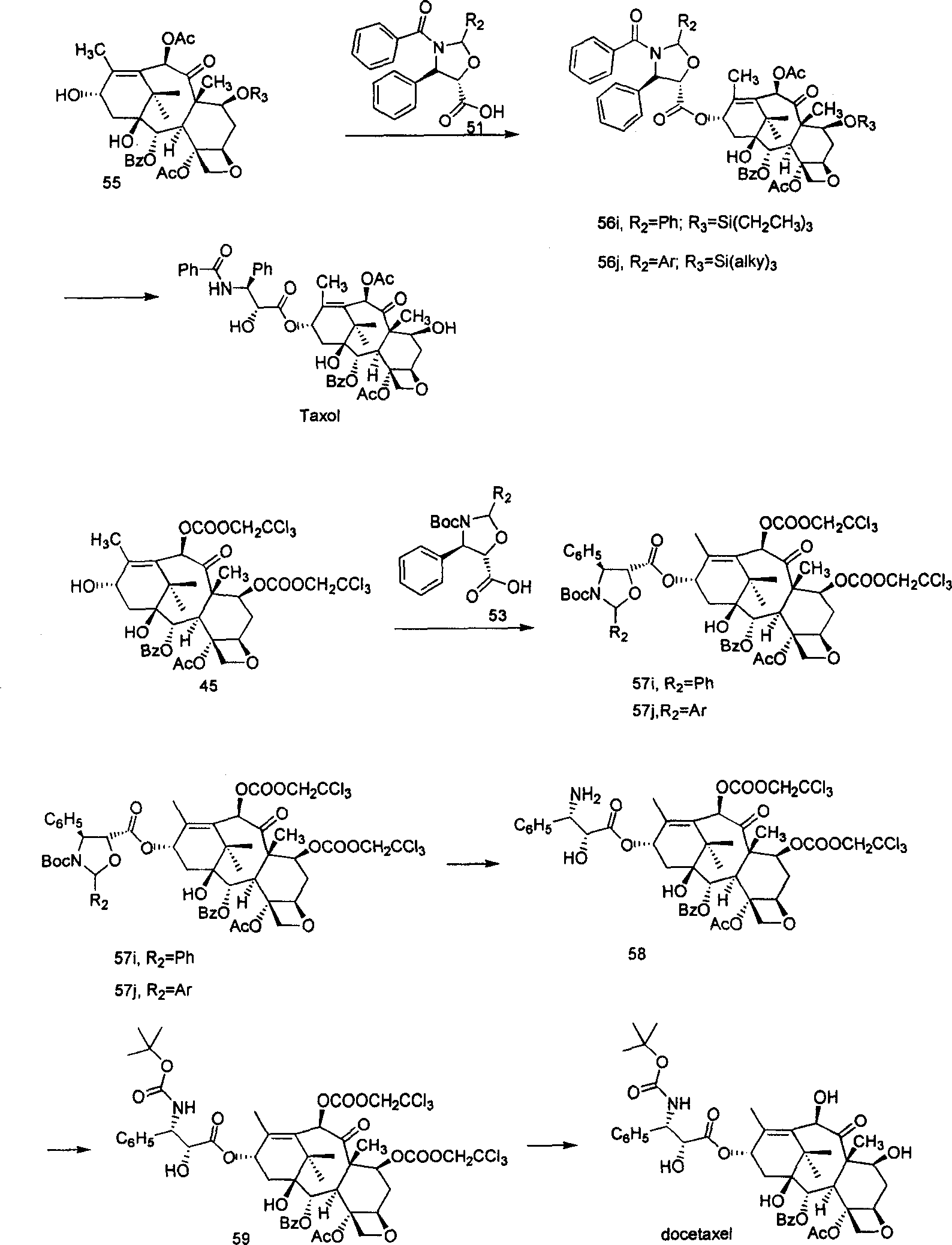
Process of synthesizing taxol and docetaxel
A technology of docetaxel and synthesis method, which is applied in the field of synthesis of paclitaxel and docetaxel, can solve the problem of expensive baccatin III, and achieve the effect of high optical purity and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
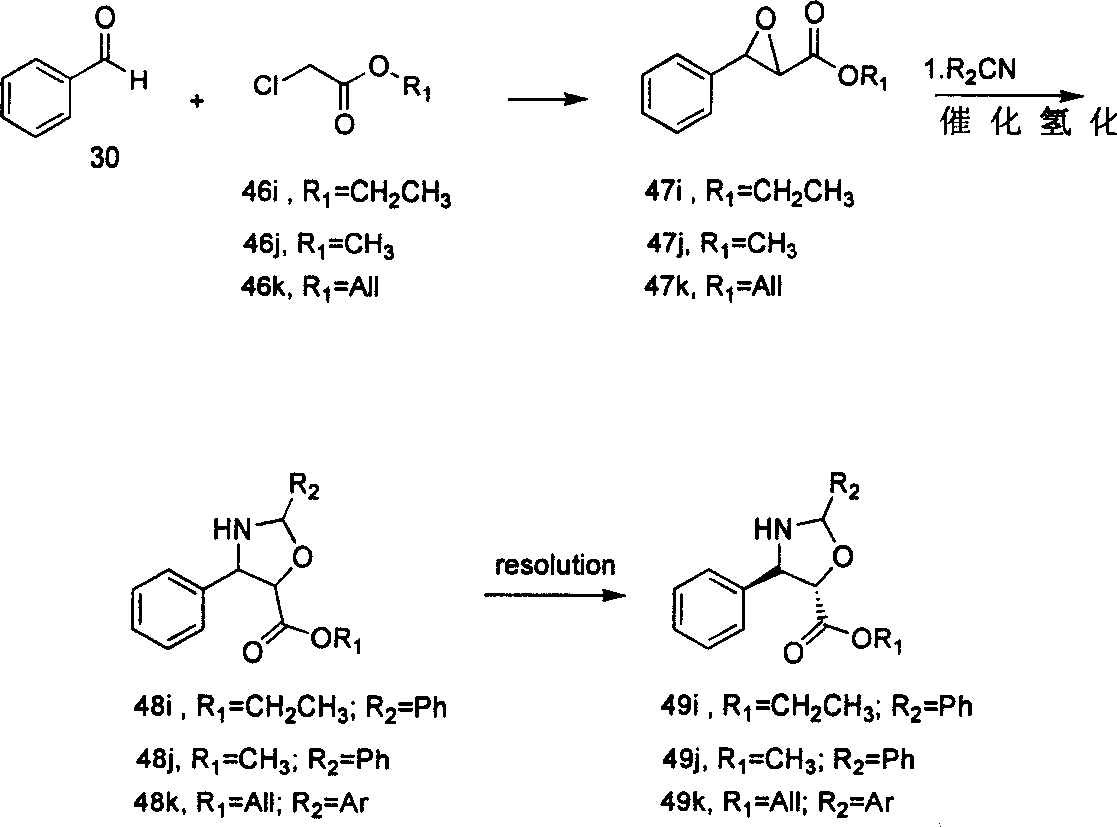
[0091] Preparation of compound 47i
[0092]
[0093] Sodium ethoxide (0.70g, 10.36mmol) was added to 10mL of ethanol, stirred slowly, and an ethanol solution of benzaldehyde (0.96mL, 9.42mmol) was added dropwise under ice-cooling. After the addition was completed, it was raised to room temperature to continue the reaction. After cooling in a bath, ethyl chloroacetate (1.1 mL, 10.36 mmol) was added dropwise. After the dropwise addition, it was warmed to room temperature and tracked by TLC. After 12 hours, the reaction was complete. Added 100 mL of deionized water and extracted with ethyl acetate (50 mL×3). The organic layers were combined, dried over anhydrous sodium sulfate, filtered, and the filtrate was distilled under reduced pressure to obtain a colorless oil 47i (1.63 g, 90.1% yield).
[0094] Colorless oil; IR υ max : 3015, 2978, 2950, 2919, 2880, 1730, 1455, 1360, 1046cm -1 ; MSm / z (%): 192, 176, 146, 135, 118.
[0095] 1 H NMR (300MHz, CDCl 3 )δ7.24 (5H, m, P...
Embodiment 2
[0097] Preparation of compound 48i
[0098]
[0099]Dissolve compound 47i (0.72g, 3.75mmol) in 10mL of benzonitrile, add boron trifluoride ether solution (2equiv.), stir at room temperature, and detect by TLC. After 3h, the reaction is complete. The solvent is evaporated under reduced pressure, and the residue is dissolved in In methanol, add 0.05g containing 10% Pd / C, pass hydrogen, stir at normal temperature and pressure, TLC tracking, after 11h, the reaction is complete, filter, evaporate the solvent under reduced pressure, the crude product is subjected to column chromatography to obtain colorless Oil 48i (0.92 g, 82.9% yield).
Embodiment 3
[0101] Preparation of compound 49i
[0102]
[0103] Dissolve the racemic compound 48i (0.60g, 2.02mmol) in 10mL of toluene, add 0.03g of Novozymes lipase, and octanol (1equiv.), stir slowly, and control the temperature of the liquid in the device at about 30°C. After 26 hours, the reaction was completed, Novozymes lipase was filtered off, the filtrate was spin-dried, and the residue was distilled under reduced pressure to obtain compound 49i (0.25 g, 41.7% yield). Yellow oil; [α] D 20 =+18.0°(C1.0, CHCl 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com