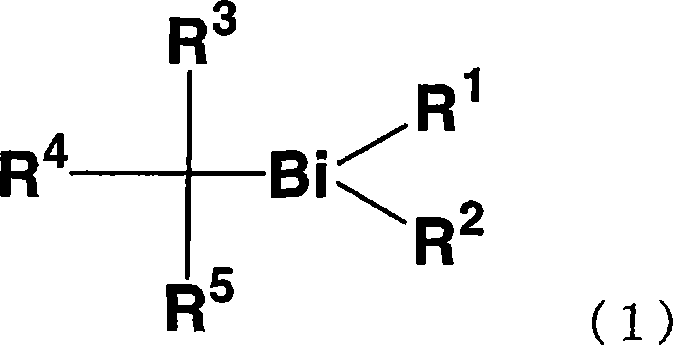
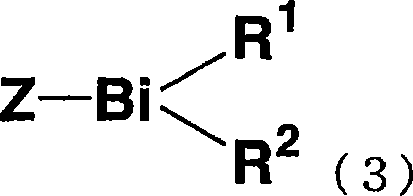
Organic bismuth compound, method for producing same, living radical polymerization initiator, method for producing polymer using same, and polymer
A technology of polymerization initiator and free radical, applied in the direction of bismuth organic compounds, etc., can solve the problems of undisclosed organic bismuth compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 4
[0184] Tetramethyldi (Synthesis Example 7)
[0185] Azo polymerization initiator:
[0186] 2,2'-Azobis(isobutyronitrile) (manufactured by Daisuke Chemical Co., Ltd., trade name: AIBN)
[0187] 1,1'-Azobis(1-cyclohexanenitrile) (manufactured by Daishin Chemical Co., Ltd., trade name: ACHN)
[0188] 2,2'-Azobis-2,4-dimethylvaleronitrile (manufactured by Daisen Chemical Co., Ltd., trade name: ADVN)
[0189] monomer:
[0190] Adamantane Monomer MADM
[0191]
[0192] Norbornane Monomer NBLM
[0193]
Synthetic example 1
[0195] Trimethyl Synthesis
[0196] Under an argon atmosphere, 25 g of bismuth tribromide dissolved in 40 ml of THF was added dropwise to 180 ml of a 1.0 M methyl lithium diethyl ether solution while maintaining reflux. It was then stirred at room temperature for 1.5 hours. After distilling off the solvent under normal pressure, distillation was performed under reduced pressure. By distilling the obtained distillate again, 8.1 g (54.8%) of a colorless oily substance was obtained.
[0197] pass 1 H-NMR confirmed the target object.
[0198] 1 H-NMR (300MHz, CDCl 3 )1.13(s, 9H)
Synthetic example 2
[0200] Dimethyl Synthesis of bromides
[0201] Under the atmosphere of argon gas, the trimethyl group synthesized in Synthesis Example 1 A liquid in which 7.2 g of bismuth tribromide was dissolved in 13 ml of THF was added dropwise to 8.0 g (31.5 mmol). It was then stirred at room temperature for 1.5 hours. After the reaction solution was filtered through a 0.2 µm membrane filter, THF was distilled off under reduced pressure to obtain 14.23 g (94.5%) of a light yellow amorphous substance.
[0202] pass 1 H-NMR confirmed the target object.
[0203] 1 H-NMR (300MHz, DMSO) 1.52(s, 6H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com