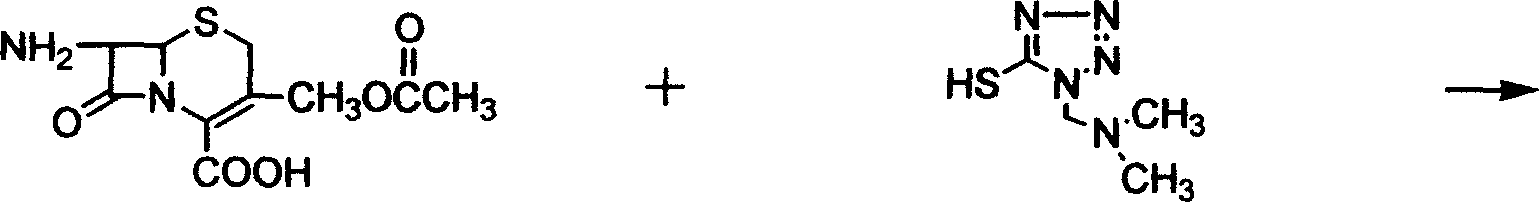Method for preparing cefotiam dihydrate dihydrochloride
A technology of cefotiam and dihydrochloride, which is applied in the field of preparation of cefotiam dihydrate dihydrochloride, can solve the problems of high product color grade, high impurity residue, poor reaction degree, etc., and achieve excellent color, The effect of less residual impurities and high content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
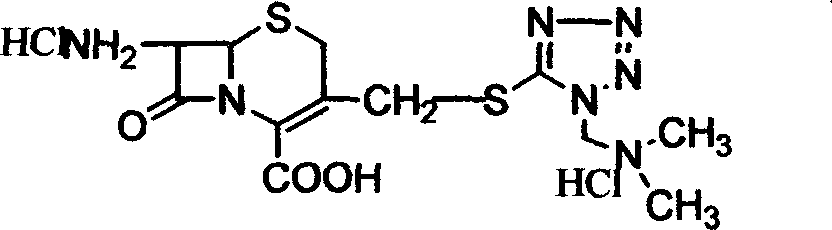
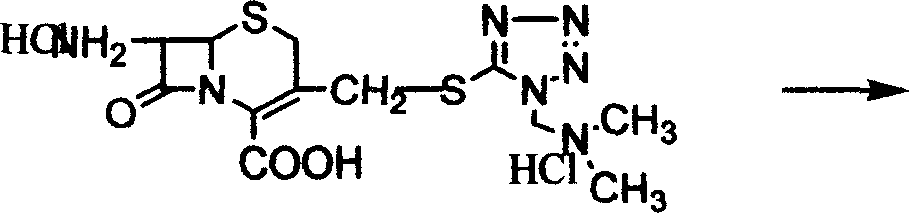
[0032] Add acetonitrile, 31.54 g of 7-aminocephalosporanic acid, [(N,N)-dimethyl-amino-ethyl]-5-mercapto-tetrazolium at room temperature, and add trifluoride Boron-acetonitrile complex 185ml, time 5 hours when the temperature is raised to 20°C, add acetone, when the temperature is 18°C, add 37% hydrochloric acid and acetone dropwise, then add pre-cooled acetone, extract the material, wash with acetone, and obtain wet Taste. Add the wet product from the previous step to methanol and water, add carbon, stir, filter with suction, and wash with a mixture of methanol and water. Add triethylamine dropwise to the filtrate at a temperature of 15±5°C, then add methanol and water until pH = 3, stir for 1 hour at a temperature of 10°C, extract the material, wash with methanol and acetone successively, and drain to obtain [ 3-[[[(N,N)-Dimethyl-amino-ethyl-tetrazol-1-yl]thio]methyl]-8oxo-5-thia-1H-azabicyclo[4 , 2,0]oct-2-ene-2-carboxylic acid] hydrochloric acid solid 39.9g, high perform...
Embodiment 2
[0034] Add ether, 31.54g of 7-aminocephalosporanic acid, [(N,N)-dimethyl-amino-ethyl]-5-mercapto-tetrazolium, after complete dissolution, add boron trifluoride at 5°C -Ether complex 185ml, timed for 5 hours at a temperature of 20°C, sampling and measuring the 7-aminocephalosporanic acid content of 4.48%. Add acetone, drop hydrochloric acid and acetone when the temperature is 18°C, then add pre-cooled acetone. Stir for 30 minutes at a temperature of 10° C., extract the material, and wash with acetone to obtain a wet product. Add the wet product from the previous step to methanol and water, add carbon and stir for 30 minutes, filter with suction, and wash with a mixture of methanol and water. When the temperature of the filtrate is 15±5°C, add triethylamine, methanol and water dropwise, stir for 1 hour at a temperature of 10°C, extract the material, wash with methanol and acetone successively, and drain to obtain [3-[[[(N , N)-Dimethyl-amino-ethyl-tetrazol-1-yl]thio]methyl]-8 ...
Embodiment 3
[0036] At room temperature, add acetonitrile, dimethyl carbonate, 31.54 g of 7-aminocephalosporanic acid, [(N,N)-dimethyl-amino-ethyl]-5-mercapto-tetrazolium, and dissolve them completely at temperature Add 185ml of boron trifluoride-dimethyl carbonate complex at 5°C, control the temperature at 20°C and measure it online. When the content of 7-aminocephalosporanic acid is 4.84%, add acetone, and drop Add hydrochloric acid and acetone, then add pre-cooled acetone. Stir at 10°C for 30 minutes, extract the material, and wash with acetone to obtain a wet product. Add the wet product from the previous step to methanol and water, add carbon, stir for 30 minutes, filter with suction, and wash with a mixture of methanol and water. When the filtrate temperature is 15±5°C, add triethylamine, methanol 1, and water dropwise, stir at 10°C for 1 hour, take out the material, wash it with methanol and acetone successively, and dry it to obtain [3-[[[(N , N)-Dimethyl-amino-ethyl-tetrazol-1-y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com