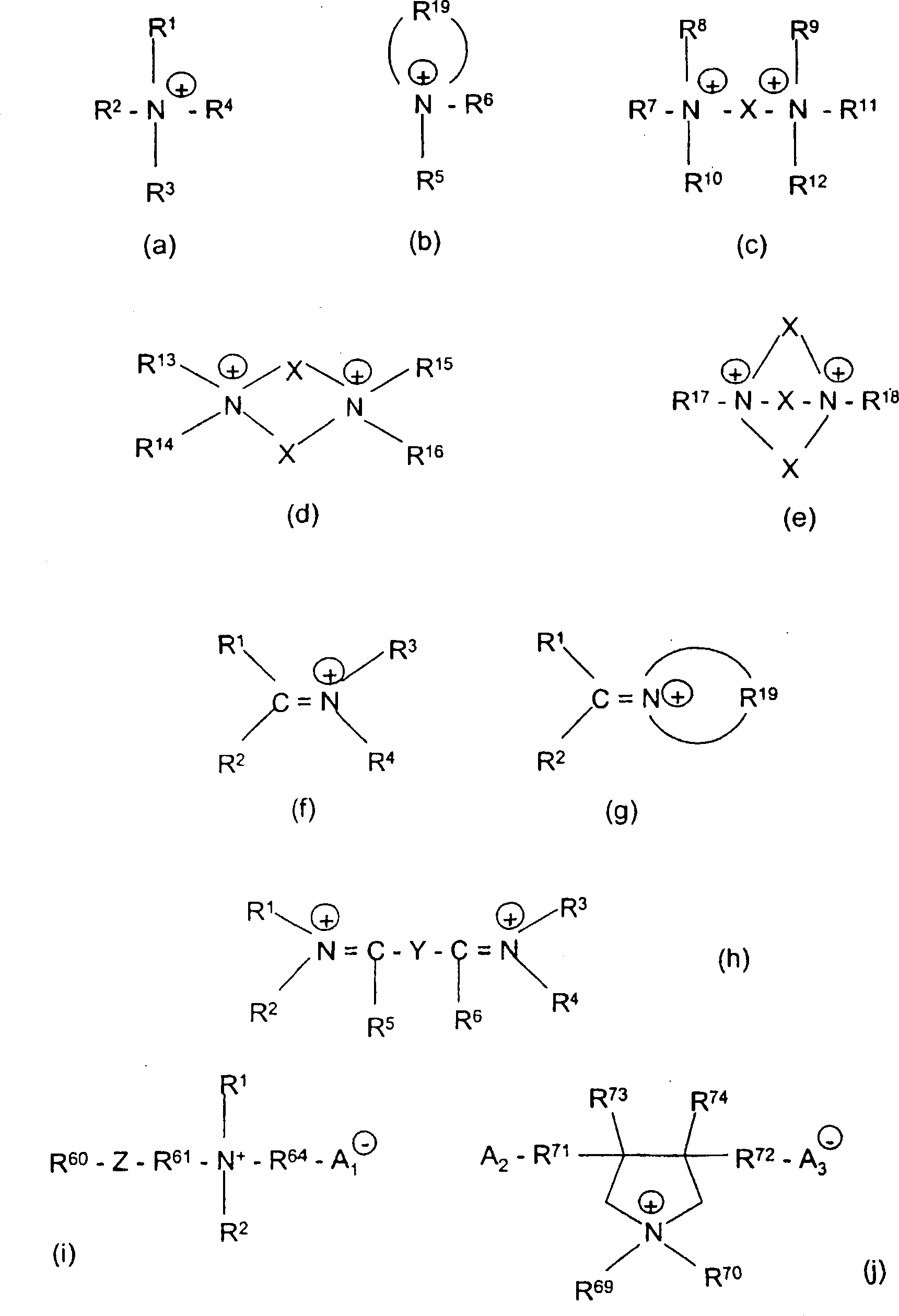
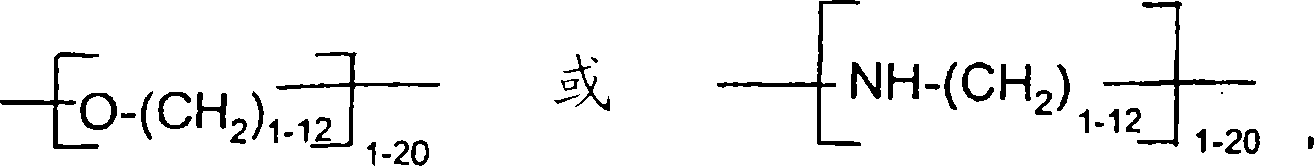
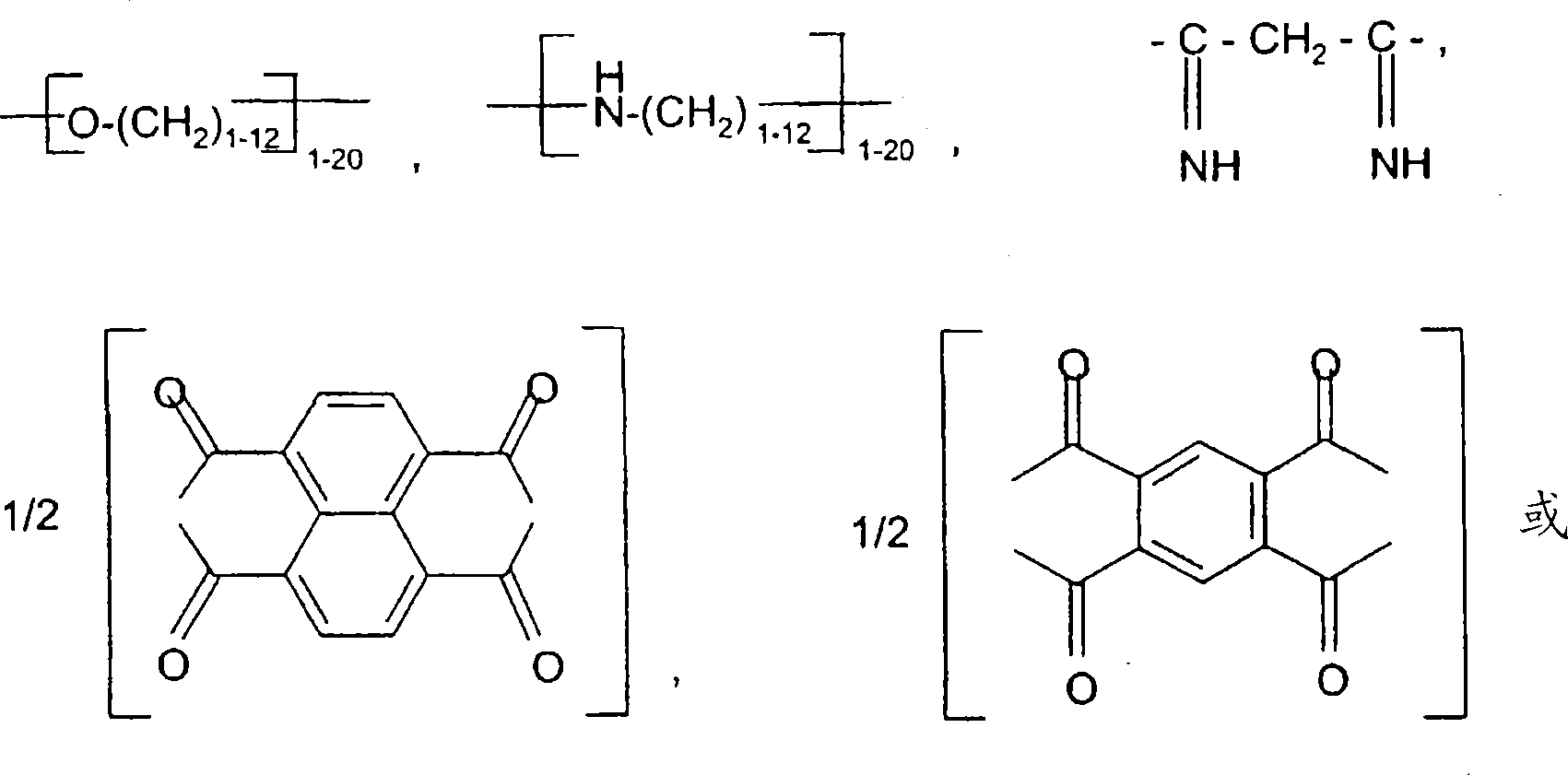
Hydrophobic, salt-like structured silicate
A structural, silicate technology, applied in inorganic chemistry, silicon compounds, fibrous fillers, etc., to solve problems such as not achieving uniform distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0100]25 g of bentonite (pH 7-12) were dispersed in 500 ml of deionized water by stirring at 20° C. for 12 hours. The suspension was then adjusted to a pH value of 4-10 by dilute sulfuric acid and thereafter 10 g of a 77% aqueous solution of distearyl dimethyl ammonium chloride (DSDMAC) was added to the bentonite suspension, and the reaction mixture was heated at 60° C. Stir for 1 hour. After a reaction time of 1 hour, a mixture of 7 g of a 77% strength aqueous solution of DSDMAC and 50 g of a 10% strength aqueous montanic ester wax dispersion prepared by adding 10 g of molten montanic ester wax ((R) Licowax F, Clariant Company, acid Value 6-10mg KOH / g, drop point 75-81 ℃) add about 95 ℃ hot water solution preparation, this hot water solution is prepared by 0.7g 21% concentration KOH-ethylene glycol solution, 3g 10% concentration polyvinyl alcohol solution (( ) Mowiol 4-88, Kuraray Company, Germany) and 86.3g deionized water.
[0101] The reaction mixture was stirred at 60° ...
preparation Embodiment 2
[0113] 25 g of bentonite (pH 7-12) were dispersed in 500 ml of deionized water by stirring at 20° C. for 12 hours. The suspension was then adjusted to a pH value of 4-10 by dilute sulfuric acid and thereafter 17 g of 77% strength distearyl dimethyl ammonium chloride aqueous solution (DSDMAC) was added in two portions to the bentonite suspension, and the reaction mixture Stir at 80°C for 1 hour. After a reaction time of 1 hour, 50 g of a 10% strength isopropanol wax solution comprising a wax mixture of 75% erucamide wax and 25% carnauba wax were added. The reaction mixture was stirred at 80° C. for a further 1 hour, and the solid was filtered off with suction, post-washed several times with deionized water and then dried at 60° C. in vacuo.
[0114] Yield: 38.9 g of ivory powder.
[0115] Characterization:
[0116] Appearance: ivory powder
[0117] DTA: No decomposition detected until 400°C
[0118] pH: 7.5
[0119] Conductivity: 0.18mS / cm
[0120] Residual moisture: 1.1...
preparation Embodiment 3
[0127] 25 g of bentonite (pH 7-12) were dispersed in 500 ml of deionized water by stirring at 20° C. for 12 hours. The suspension was then adjusted to a pH value of 4-10 by dilute sulfuric acid and thereafter 17 g of 77% strength distearyl dimethyl ammonium chloride aqueous solution (DSDMAC) was added in two portions to the bentonite suspension, and the reaction mixture Stir at 80°C for 1 hour. After a reaction time of 1 hour, an aqueous aluminum stearate dispersion was added by dissolving 5 g of stearic acid, 95 g of deionized water, 1.8 g of sodium hydroxide pastilles, 8 g of isopropanol and 0.5 g of Coco fatty alcohol polyglycol ether ((R) Genapol C 050, Clariant Company, Germany), followed by 2.3 g of Al in 50 g of deionized water at the same temperature 2 (SO 4 ) 3 18H 2 A solution of O was prepared for precipitation and adjusting the suspension of the precipitate to pH 3-12. The reaction mixture was then adjusted to a pH of 3-10, stirred at 80° C. for a further 1 ho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Dropping point | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com