Novel crystal of doripenem, preparation method and use thereof
A doripenem and crystallization technology, which is applied in the field of application and pharmaceutical compositions containing new crystal forms of doripenem, can solve the problems of high preparation cost, discoloration, and complicated preparation process, and achieve the effect of low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] [Example 1] Preparation of crude product of doripenem:
[0042]Doripenem can be synthesized by previously known methods. Such as using the method disclosed in CN200510021270.1 to obtain the crude product of doripenem. It can also be prepared by:
[0043] (1), preparation of mercaptopyrrolidine derivatives
[0044] (2S, 4S)-1-p-nitrobenzyloxycarbonyl-2-(N-p-nitrobenzyloxycarbonyl-N-sulfamoylamino)methyl-4-acetylthiopyrrolidine 50 g (81.8 mg mol) was dissolved in 200 milliliters of tetrahydrofuran, and 20 milliliters of aqueous solution of 6 grams of lithium hydroxide was added dropwise under an ice bath. After the addition was completed, stirring was continued for 120 minutes, acidified with 6 equivalents of hydrochloric acid, and a solid viscous substance was precipitated, which was washed with ethyl acetate Add ethanol after dissolving, freeze and precipitate 32 grams of light yellow amorphous solid powder, yield 68.8%.
[0045] (2), preparation of protected pyrrol...
Embodiment 2
[0049] [Example 2] Preparation of new crystals of doripenem of the present invention
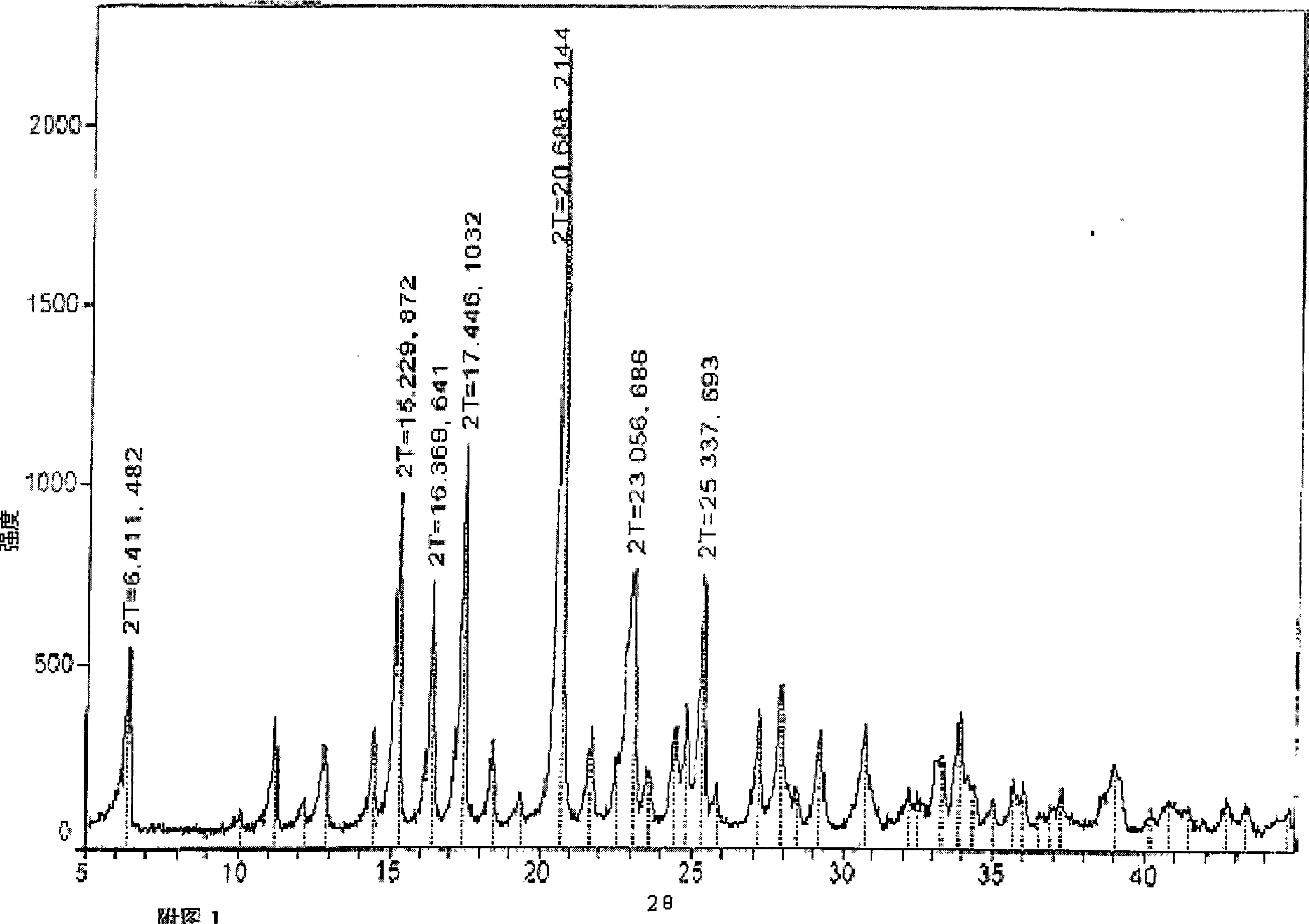
[0050] 1. Preparation of seed crystals: Add 10 grams of crude doripenem into 100 ml of distilled water, extract 3 times with 40 ml of ethyl acetate, place the aqueous solution at room temperature (8-13°C) overnight, precipitate crystals, filter, Dry under vacuum at room temperature to obtain seed crystals.
[0051] 2. Preparation of new crystals: add 10 g of crude doripenem to 200 ml of distilled water, heat to dissolve at 50±5°C, add 0.5 g of needles and decolorize with activated carbon for 10 minutes, filter, and cool the filtrate to 0-5°C Finally, 20 mg of V-type crystal seed crystals were added, stirred for 4 hours to precipitate the crystals, and then slowly added dropwise with 100 ml of isopropanol. After the dropwise addition, the temperature was lowered to -10°C to continue crystallization and aging overnight, and then the crystals were filtered out. The obtained crystals were washe...
Embodiment 3
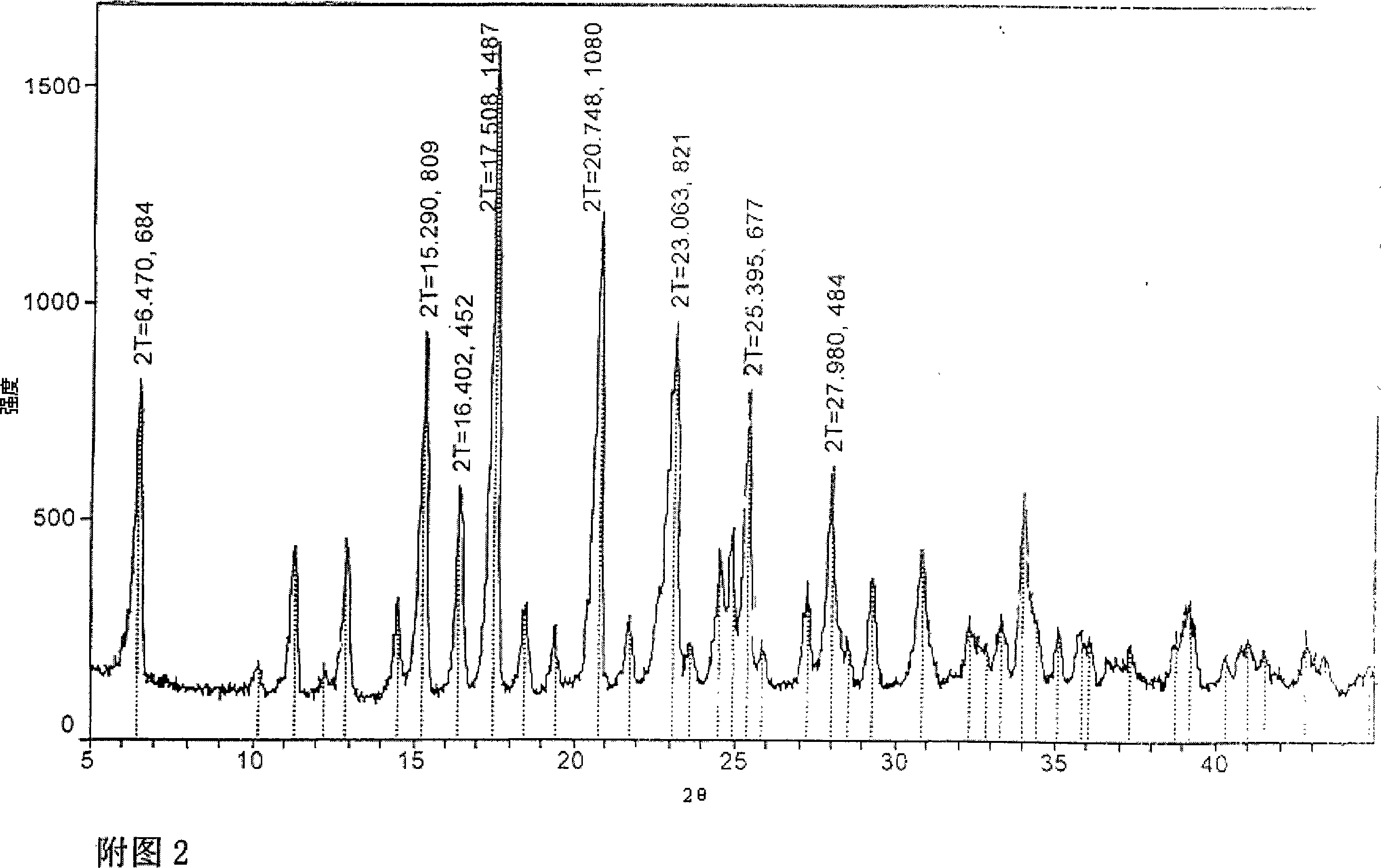
[0066] [Example 3] Repeated preparation of new crystals of doripenem of the present invention:
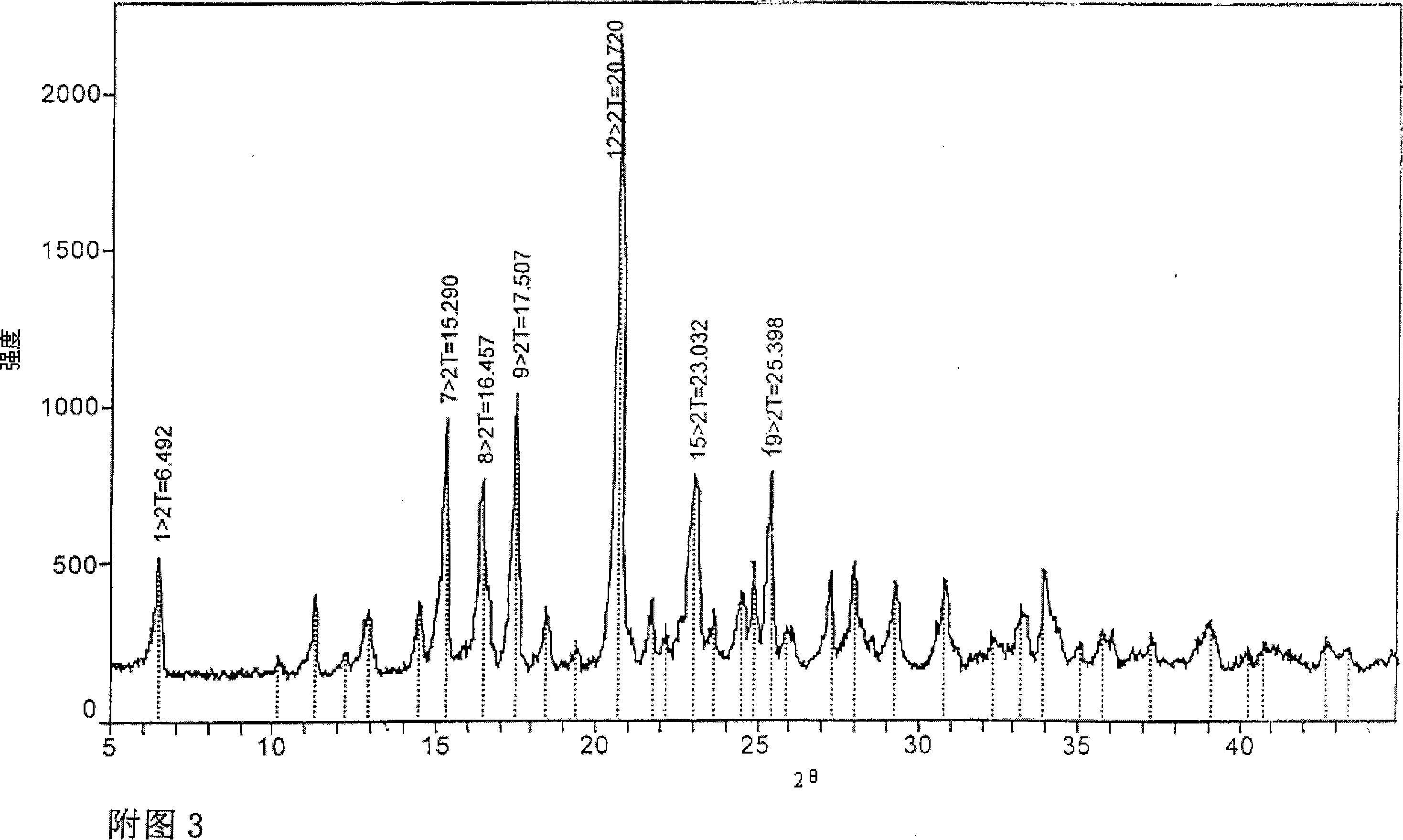
[0067] In order to confirm the repeatability of the above-mentioned Example 1, the V-type crystal obtained in Example 2 was used as the seed crystal to carry out repeated tests. The powder X-ray diffraction measurement result of the obtained crystal placed for one month is shown in Fig. 2, and there are main peaks at diffraction angles (2θ)=6.471, 15.290, 16.402, 17.508, 20.748, 23.063 and 25.396 (degrees). In addition, there are lower peaks at the diffraction angle (2θ) = 11.275, 12.224, 12.894, 14.506, 18.444, 19.375, 21.739, 23.661, 24.528, 24.890, 25.911, 27.256, 27.981, 29.269, 30.804, 33.929, 39. .
[0068] Moisture content: theoretical value (dihydrate): 7.89%, measured value by thermogravimetric analysis (TGA): 8.003%.
[0069] The melting point measured by differential scanning calorimetry (DSC) is 175.71° C.; the infrared spectrum data and nuclear magnetic resonance dat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com