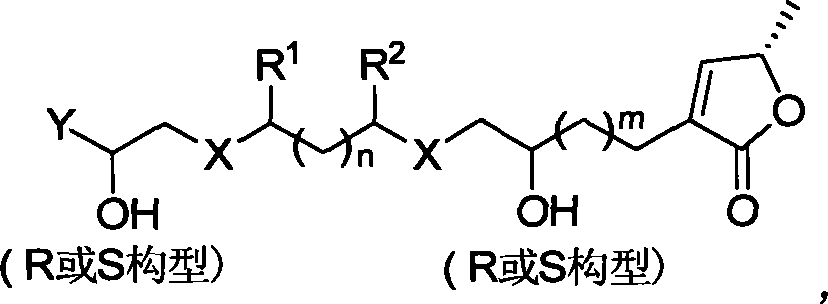
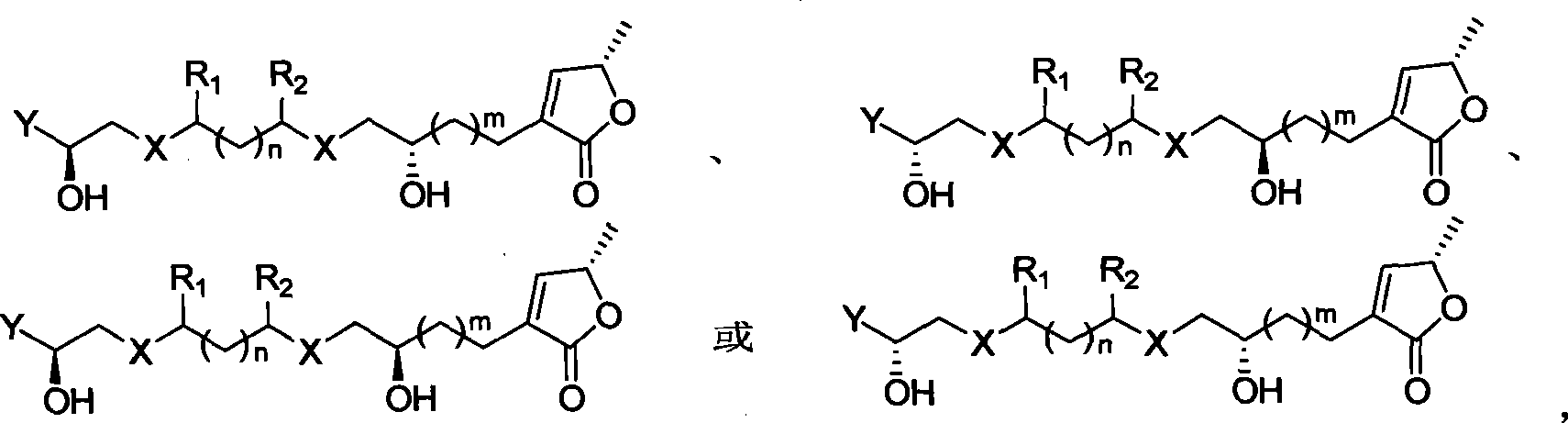
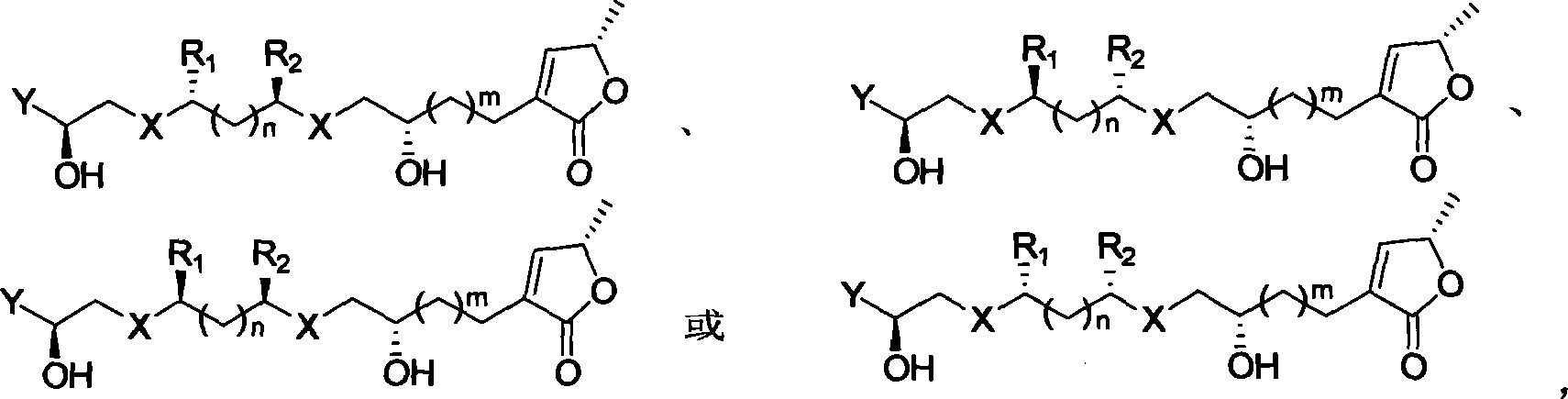
Chirality anona squamosa L. lactone compound with conformation limitation structure and synthesizing method and use thereof
A kind of annua lactone, a technology of limiting structure, applied in the field of chiral annua lactone analogs and their synthesis, and can solve the problems such as not obtaining chiral products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056]
[0057] Dissolve 4.76mmol of 60% sodium hydride in 10mL of DMF, cool to 0°C and slowly drop into 15mL of the raw material 4.76mmol of diol in DMF, stir at this temperature for 30 minutes, then slowly drop into 15mL of the raw material 2.38mmol of 14 DMF solution, the temperature of the reaction solution was slowly raised to 80° C. after dropping, and reacted for 15 hours. Cool to room temperature, quench with saturated ammonium chloride, dilute the system with 150mL ethyl acetate, separate the organic phase, wash with water, saturated ammonium chloride and saturated sodium chloride successively, dry over anhydrous sodium sulfate, filter, concentrate, and obtain by column chromatography Pale yellow liquid 16 (0.53 g, 82%).
[0058] [α] D 25 =+3.6(c 3.1, CHCl 3 );
[0059] 1 H NMR (400MHz, CDCl 3 )δ: 0.88 (3H, t, J=7.0Hz), 1.23 (16H, m), 1.52 (2H, m), 3.37 (3H, s), 3.38 (3H, s), 3.39 (3H, s), 3.53-3.78 (8H, m), 3.87 (1H, dd, J = 5.5, 10.0Hz), 4.64 (5H, m), 4.75...
Embodiment 2
[0063]
[0064] Add 0.21 g of tetrabutylammonium bisulfate as a phase transfer catalyst to the 40 mL cyclohexane solution of raw materials 4.8 mmol 16 and 9.6 mmol epichlorohydrin, stir evenly and cool to 0 ° C, slowly add 10 mL of 50% NaOH aqueous solution dropwise, at room temperature After stirring at low temperature for 10 hours, the system was diluted with 100 mL ether, the organic phase was separated, washed with water and saturated sodium chloride successively, dried over anhydrous sodium sulfate, concentrated, and column chromatography gave colorless liquid 17 (1.42 g, 60%).
[0065] [α] D 25 =-15.5(c 2.1, CHCl 3 );
[0066] 1 H NMR (500MHz, CDCl 3 )δ: 0.88 (3H, t, J = 7.0Hz), 1.26 (16H, m), 1.55 (2H, m), 2.60 (1H, m), 2.77 (1H, dd, J = 4.2, 5.0Hz), 3.15(1H,m), 3.36(6H,m), 3.37(3H,s), 3.54-3.76(10H,m), 3.84(1H,m), 4.63(5H,m), 4.77(1H,d, J = 6.7Hz) ppm;
[0067] 13 C NMR (100MHz, CDCl 3 )δ: 96.7, 96.6, 95.9, 79.38, 79.32, 76.4, 73.8, 72.2, 72.0, 67.1, 66.8, ...
Embodiment 3
[0071]
[0072] -78°C and under the protection of nitrogen, slowly drop 4.38mmol n-BuLi (1.6M in hexane) into 4.38mmol trimethylsilylacetylene in 5mL anhydrous tetrahydrofuran, stir for 30 minutes, then add 4.38mmol BF 3 ·Et 2 O, after continuing to stir for 30 minutes, slowly drop into 15mL of anhydrous tetrahydrofuran solution of compound 1.46mmol 17, after 2 hours of reaction, quench with saturated ammonium chloride, dilute the system with 50mL of ether, separate the organic phase with saturated sodium bicarbonate and Wash with saturated sodium chloride, dry over anhydrous sodium sulfate, concentrate to obtain the intermediate, dissolve in 10 mL of anhydrous dichloromethane, add 21.9 mmol of diisopropylethylamine at room temperature, cool to 0 ° C, slowly inject 14.6 mmol of MOMCl, React at room temperature for 5 hours, quenched with saturated sodium bicarbonate, dilute the system with 50 mL of dichloromethane, separate the organic phase, wash with 1N HCI, saturated sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com