Bone-repairing material and preparing method thereof
A technology for bone repair and raw materials, applied in the field of bone repair materials and their preparation, can solve the problems of local tuberculosis foci abscess recurrence, shortening the course of anti-tuberculosis drugs, lack of administration methods, etc., and achieves reduction of local abscess recurrence and continuous osteogenesis ability. , good biocompatibility and bioabsorbability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
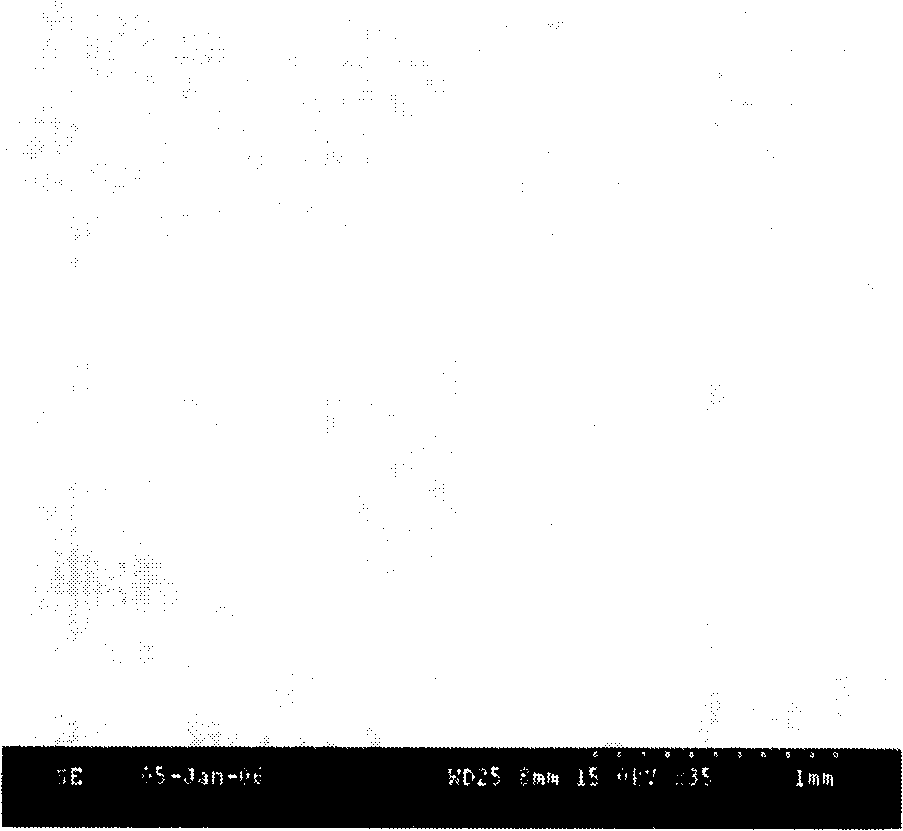
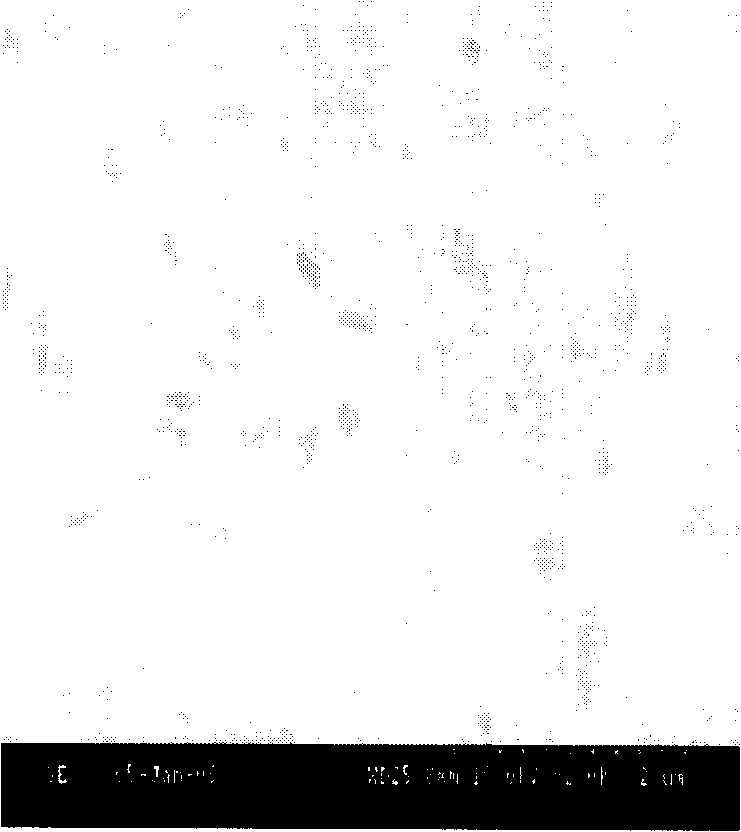
Image
Examples
Embodiment 1
[0039] 1 Materials and methods
[0040] 1.1 Materials
[0041] PLGA (85 / 15) was provided by Chengdu Organic Chemistry Co., Ltd., Chinese Academy of Sciences, with a relative molecular mass of 300,000; calcium sulfate hemihydrate crystals were provided by Wright Company; rifampicin reference substance was provided by Qingdao Sangon Biotechnology Co., Ltd.
[0042] 1.2 Method
[0043] 1.2.1 Preparation of materials
[0044] Calcium sulfate hemihydrate: PLGA: sodium chloride: rifampicin=1:1:2:0.08 (mass ratio), wherein calcium sulfate hemihydrate and PLGA are slow-release carriers, sodium chloride is a porogen, and sodium chloride The crystal particle size is 180-250 μm. The materials were sterilized with ethylene oxide separately, and the following processing was carried out in a sterile environment.
[0045] (1) Organic solvent injection molding: add quantitative PLGA to chloroform according to the formula, fully magnetically stir, and make a solution with a concentration o...
Embodiment 2
[0058] The proportion of raw materials used in the manufacture of bone repair materials in this embodiment is as follows:
[0059] PLGA: calcium sulfate hemihydrate: sodium chloride granules: rifampicin = 40:20:80:2.
[0060] The calcium sulfate hemihydrate refers to powder with a particle size below 80um; the PLGA refers to PLGA with a molecular weight of 3-100,000; the sodium chloride refers to sodium chloride crystal particles of 200-600um.
[0061] The method for manufacturing the bone repair material in this embodiment is as follows:
[0062] 1) Add the amount of PLGA into chloroform to form a 15-25% solution, seal and let stand for 10-14 hours;
[0063] 2) Add the amount of rifampicin, calcium sulfate hemihydrate, and sodium chloride into the solution and fully stir and weigh the mixture;
[0064] 3) volatilize the solvent from the mixture obtained in step 2) at room temperature, and solidify;
[0065] 4) Soak the cured product obtained in step 3) in double-distilled ...
Embodiment 3
[0077] The proportion of raw materials used in the manufacture of bone repair materials in this embodiment is as follows:
[0078] PLGA: calcium sulfate hemihydrate: sodium chloride granules: rifampicin = 40:80:20:8.
[0079] The calcium sulfate hemihydrate refers to powder with a particle size below 80um; the PLGA refers to PLGA with a molecular weight of 3-100,000; the sodium chloride refers to sodium chloride crystal particles of 200-600um.
[0080] The method for manufacturing the bone repair material in this embodiment is as follows:
[0081] 1) Add the amount of PLGA into chloroform to form a 20% solution, seal and let stand for 12 hours;
[0082] 2) Add the amount of rifampicin, calcium sulfate hemihydrate, and sodium chloride particles into the solution and stir thoroughly. Until it is evenly distributed in the solution, the solution is gelatinous at this time;
[0083] 3) The mixture obtained in step 2) was placed in a room-temperature fume hood to volatilize for 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com