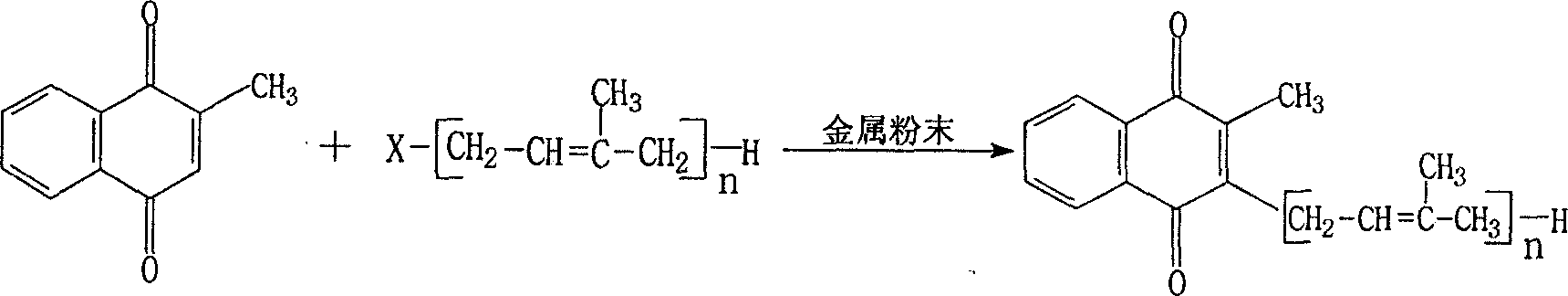
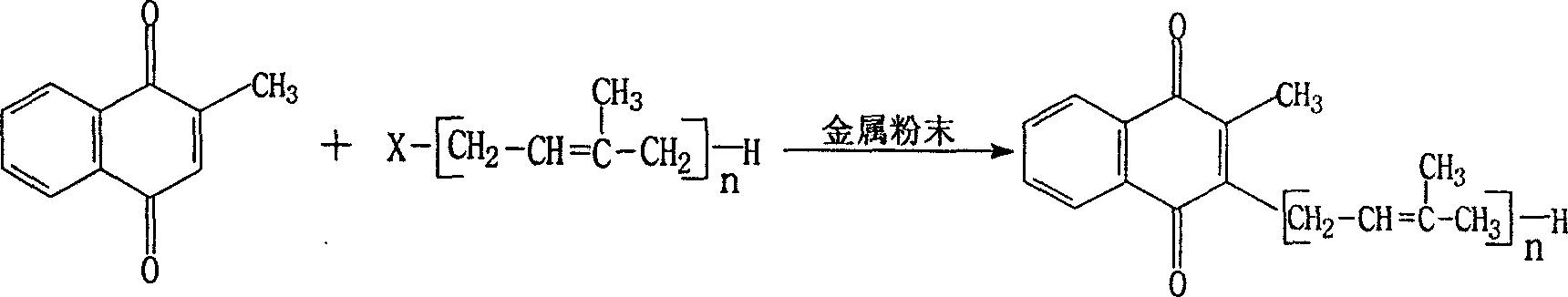
Method for synthesizing vitamin K2
A synthetic method and technology of vitamins, applied in chemical instruments and methods, preparation of organic compounds, metal/metal oxide/metal hydroxide catalysts, etc., can solve problems such as complex processes, adverse effects on product quality and yield, Achieve the effect of good product purity, beneficial to industrial production and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment one, vitamin K 2(10) preparation of
[0023] In a 1000ml three-necked flask, put 600ml of anhydrous tetrahydrofuran as a solvent, fill with nitrogen, put in 86.8 (0.4mol) of geranyl bromide, 25.6g (0.4mol) of zinc powder, and finally put in vitamin K 3 68.8g (0.4mol), heated to reflux for 6 hours while stirring, distilled off tetrahydrofuran under reduced pressure, added 400ml of hexane, and filtered off the insoluble matter in hexane. Then the hexane solution was concentrated, and the residual oil was chromatographed on silica gel, eluted with hexane:ethanol=1:1 solution, and VK was isolated 2 (10) 91.8g, yield 74.5%, purity (HPLC): 98.6%. Confirm no VK 3 Check out.
Embodiment 2
[0024] Embodiment two, vitamin VK 2(20) preparation of
[0025] Put 400ml of anhydrous tetrahydrofuran into a 1000ml three-necked flask, add 12.8g (0.2m) of zinc powder, 124.0g (0.2ml) of geranylgeranyl bromide, fill with nitrogen, and add vitamin K as soon as possible 3 34.4g (0.2mol), heated to reflux for 5 hours while stirring, and the reaction was completed. Evaporate tetrahydrofuran under reduced pressure, add hexane to filter to remove insoluble matter, concentrate the hexane solution, and subject the oil to silica gel column chromatography, eluting with ethanol: hexane = 1: 1 to obtain Vitamin K 2(20) 52.3g, yield 58.8g%, purity (HPLC): 98.2%, confirmed no VK 3 Check out.
Embodiment 3
[0026] Embodiment three, vitamin K 2(45) preparation of
[0027] In a 1000ml three-necked flask, put 400ml of anhydrous isopropyl ether, add tin powder 35.8g (0.2mol), and solanyl bromide 139.0g (0.2mol), fill with nitrogen, and add VK as soon as possible 3 34.4g (0.2mol), heated to reflux for 6 hours, the reaction was completed, distilled off isopropyl ether under reduced pressure, added hexane, filtered to remove insoluble matter, concentrated hexane solution, the oil was subjected to silica gel column chromatography, and ethanol: hexane Alkane = 1:1 elution to get vitamin K 2(45) 88.7g, yield 56.6g%, purity (HPLC): 98.3%, VK 3 ≤0.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com