Method for preparing difluorine oxalic acid boracic acid lithium
A technology of lithium difluorooxalate borate and oxalate, which is applied in the field of electrolyte salt manufacturing, can solve problems affecting battery power characteristics, poor low-temperature performance, large resistance, etc., achieve good low-temperature cycle performance, improve high-temperature cycle performance, and good The effect of battery rate capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Lithium difluorooxalate borate was prepared from boron trifluoride ether solution and lithium oxalate.
[0015] Step 1. Add 101.9g of lithium oxalate dried at 120°C for 4 hours in a dry reactor equipped with an electric stirrer and a thermometer, slowly drop in 158g of boron trifluoride ether solution, heat and stir to mix the raw materials as much as possible, and then seal The reactor was heated to 80°C for 48 hours at a constant temperature to fully react boron trifluoride and lithium oxalate to generate lithium difluorooxalate borate and lithium fluoride, and then cooled to room temperature.
[0016] Step 2, filter the reacted mixture at room temperature to remove unreacted lithium oxalate and lithium fluoride solids generated after the reaction, extract the mother liquor several times with dimethyl carbonate, concentrate under reduced pressure, cool and crystallize, and then use dicarbonate Methyl esters are subjected to a recrystallization process to remove residu...
Embodiment 2
[0021] Lithium difluorooxalate borate was prepared from lithium hydroxide, oxalic acid and boron trifluoride ether solution.
[0022] Step 1. Dissolve 47.9g of lithium hydroxide in distilled water, gradually add 140g of oxalic acid crystals, start the stirring device at the same time, slowly drop in 173.1g of boron trifluoride ether solution after it is completely dissolved, seal the reactor, and heat the whole mixture to 86° C. and kept at constant temperature for 54 hours to fully react, then cooled to room temperature.
[0023] Step 2: filter the reacted mixture at room temperature, extract the mother liquor several times with dimethyl carbonate, concentrate under reduced pressure, cool and crystallize, and then carry out recrystallization process with acetonitrile solution to remove residual impurities.
[0024] Step 3: The recrystallized product was dried at 180° C. for 48 hours under a vacuum degree of −0.08 MPa to finally obtain 129.6 g of the product, whose purity was ...
Embodiment 3
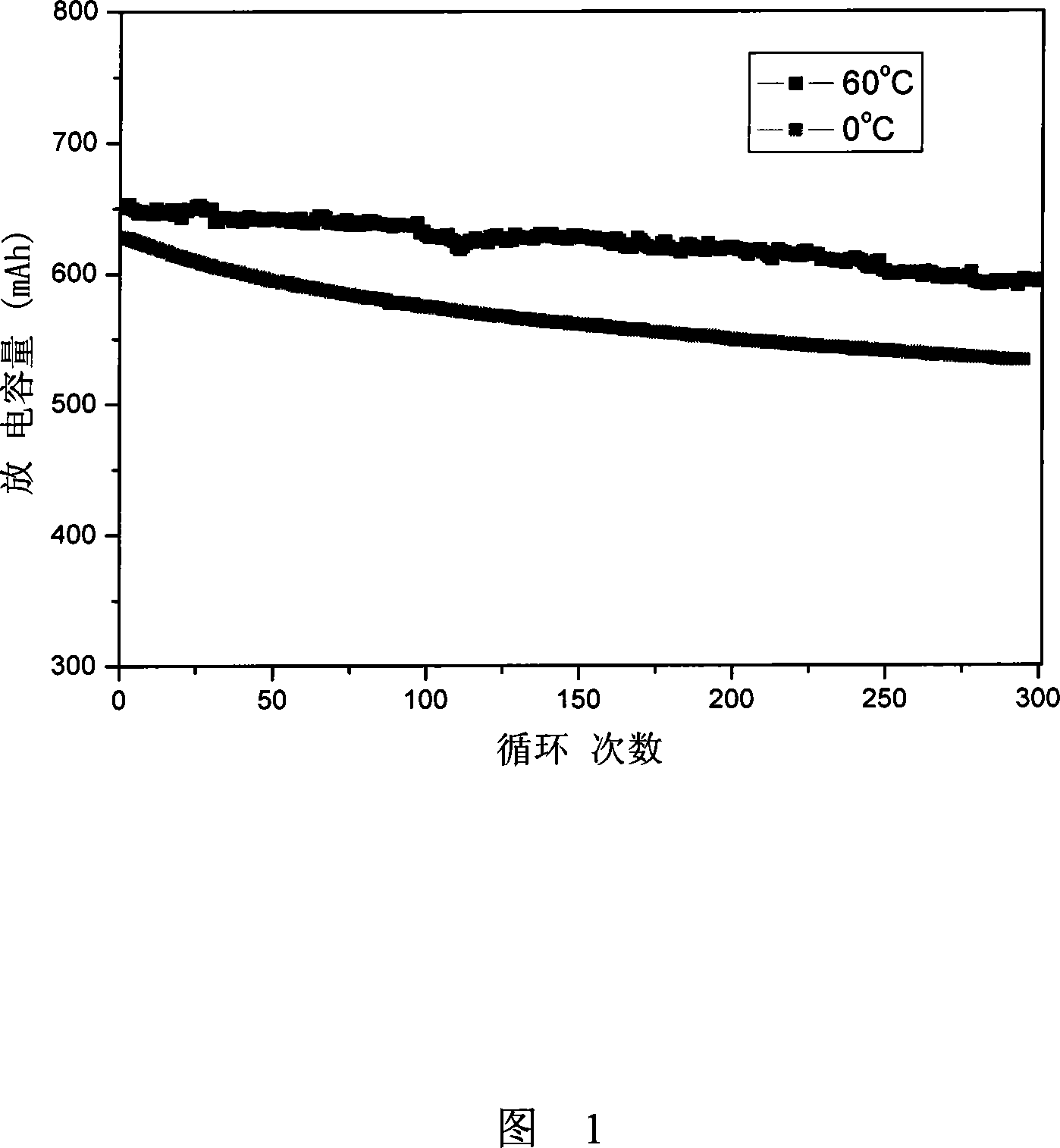
[0028] Take LiMn 2 o 4 As the positive electrode, graphite is the lithium-ion battery assembled as the negative electrode in 1mol / L LiODFB propylene carbonate / ethylene carbonate / ethyl methyl carbonate (1: 2: 1wt%) cycle performance test results are shown in Figure 1. The analysis shows that the capacity retention rate of the lithium-ion battery is 90% after 300 cycles at 1C at 60°C, and 85.4% after 300 cycles at 1C at 0°C. It can be seen that the lithium ion battery using LiODFB as the electrolyte has good high and low temperature cycle performance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| retention rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com