Double genes knockout Listeria monocytogenes attenuation mutant and constructing method
A technology of Listeria, monocytes, applied to other methods of inserting foreign genetic material, bacteria, genetic engineering, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
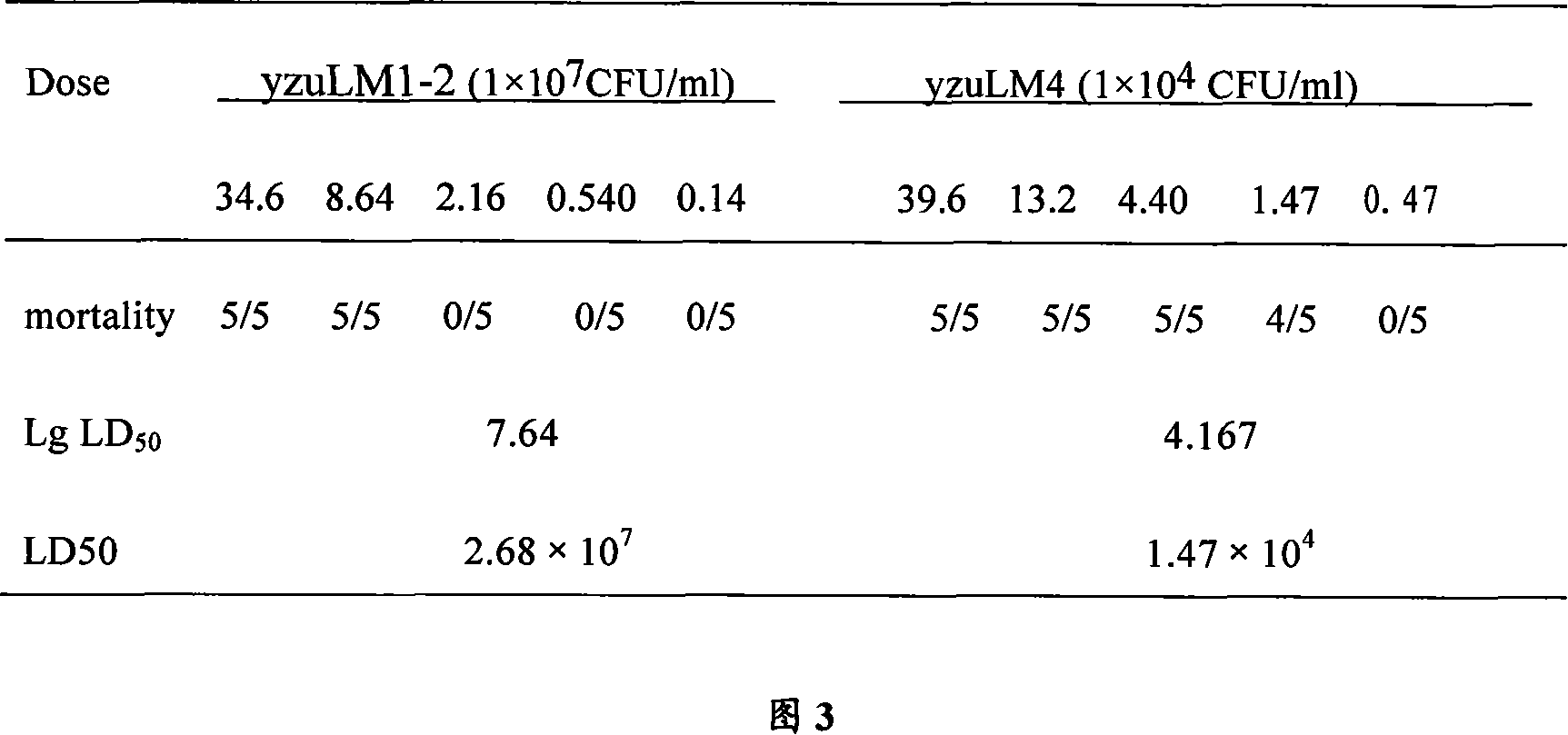
Image
Examples
Embodiment 1
[0019] The method for constructing an attenuated Listeria monocytogenes attenuated mutant strain with actA and pclB double gene deletion is characterized in that after amplifying the homologous fragments actA and plcB on both wings of the gene to be deleted, they are spliced together by SOEing PCR method, Insert into the shuttle vector pKSV7, and introduce into Listeria monocytogenes by electrotransformation, the steps are:
[0020] 1. Amplification of Homologous Fragment of ActA Gene of Listeria monocytogenes
[0021] Listeria monocytogenes LM4 was suspended in 100 μL ultrapure water at a concentration of 10 9 CFU / ml, heat at 100°C for 10 minutes, centrifuge at 12,000 rpm for 5 minutes, take 5 μL of the supernatant for PCR amplification reaction, and use the following primers:
[0022] Justice: 5'CC GAATTC TTTAGTTCCGCAGTGGATGC3'
[0023] Antisense: 5'GTCTAGCTCCAGTAGGGGATCCCTCGAGGCTGCAAATATTATG3'
[0024] The sense primer contains an ECoRI restriction site (underlined). ...
Embodiment 2
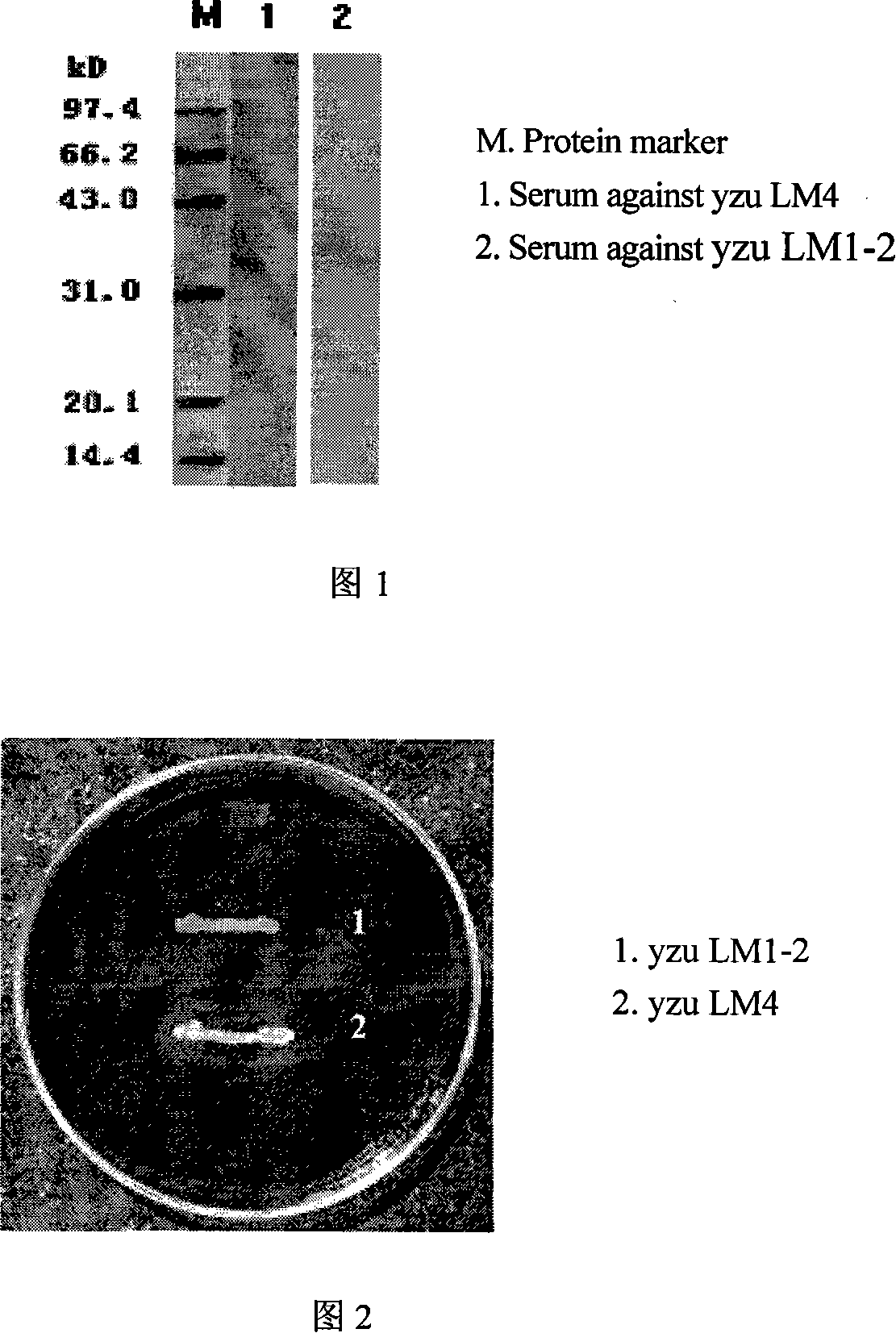
[0080] Embodiment 2: Western-blot analysis
[0081] After SDS-PAGE electrophoresis, the purified actA protein was transferred to a nitrocellulose membrane, and the polyantisera of yzu LM1-2 and LM4 were used as primary antibodies, and horseradish peroxidase-labeled goat anti-mouse IgG antibodies As the secondary antibody, diaminobenzidine (DAB) was used as the substrate for western blot analysis.
[0082] As shown in the western blot results shown in Figure 1, in lane 1, there is a specific imprint band with a size of about 97kD, but there is no imprint band in lane 2, indicating that the serum prepared from mice immunized with yzu LM1-2 does not target actA The antibody also confirmed the deletion of actA gene at the protein level.
Embodiment 3
[0083] Embodiment 3: Phospholipase activity test
[0084] Identification of biological activity: PC-PLC has the biological activity of hydrolyzing phospholipids to produce a series of water-soluble fatty acids, and egg yolk is rich in phospholipids, so the egg yolk agar method has become a convenient method for qualitatively identifying the biological activity of PC-PLC. Prepare egg yolk agar charcoal powder (YAC) medium: add 0.5 g of activated carbon powder to 100 ml of BHI solid medium, adjust the pH value to 6.5, and then autoclave it. After cooling to 45 ° C, yzu LM1-2 is scratched on the surface of the YAC medium. Line inoculated and cultured at 37°C.
[0085] After about 24 hours of cultivation on YAC medium, a transparent circle appeared around the wild-type LM4 strain inoculated on the plate. However, this phenomenon does not appear around the yzu LM1-2, as shown in Figure 2. This shows that yzuLM1-2 does not have phospholipase biological activity, thus indicating th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com