Method for synthesizing pentadecane triphenyl phosphonium salt
A technology of pentadecanetriphenyl and synthesis method, applied in the field of synthesis of pentadecatriphenylphosphine salt, can solve problems such as long reaction route, low yield, influence on large-scale production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
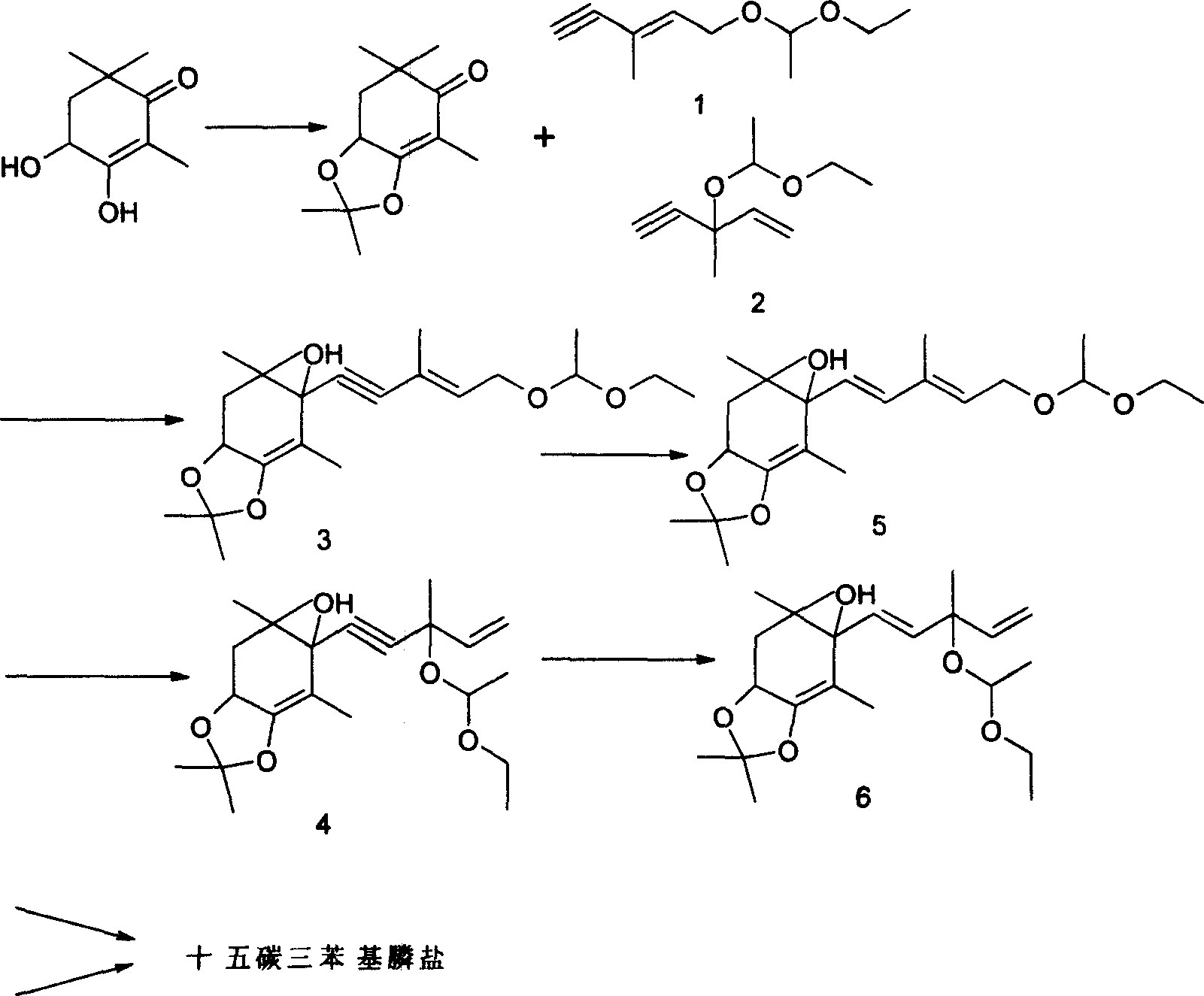
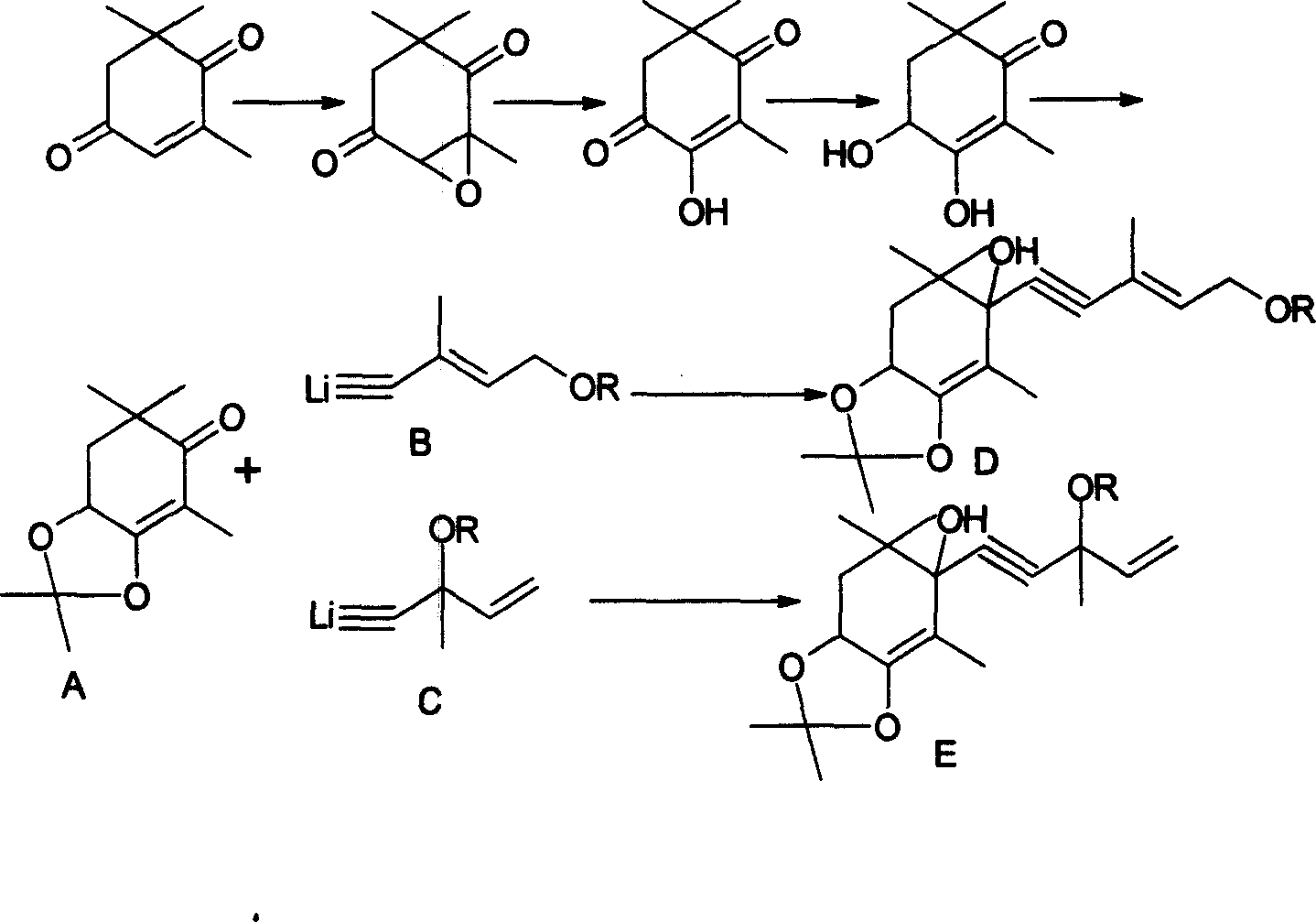
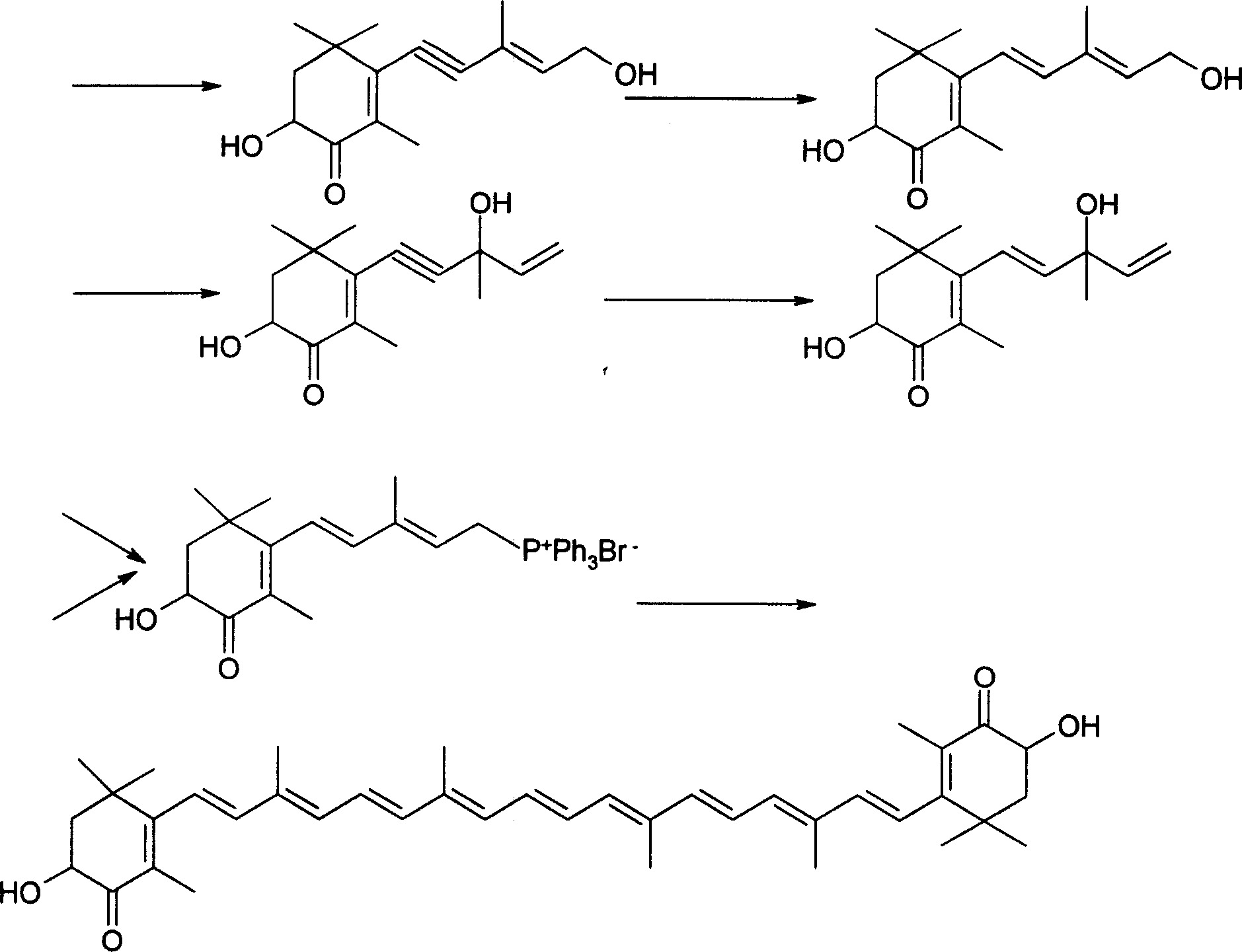
[0025] Example 1: C 9 Preparation of Units (2,2,4,6,6-Pentamethyl-7,7a-dihydro-6H-benzo[1,3]dioxol-5-one)
[0026] 170 g (1.0 mol) of crystalline 3,4-dihydroxy-2,6,6-trimethyl-2-cyclohexen-1-one were suspended in 500 ml of dichloromethane. To this suspension are initially added 500 mg (2.9 mmol) p-toluenesulfonic acid and then 144 g (2.0 mol) vinyl ethyl ether at room temperature (RT) over a period of 2 hours. The mixture was then stirred at room temperature for 4 h, after which 100 ml of 5% strength sodium hydroxide solution were added. The lower organic phase was separated off, the aqueous phase was extracted once with 100 ml of dichloromethane, the combined organic phases were washed with 200 ml of water and concentrated on a rotary evaporator. The residue was dried under reduced pressure (oil pump) to give 2,2,4,6,6-pentamethyl-7,7a-dihydro-6H-benzo[1,3]dioxolane En-5-one, as a yellow oil, was pure by thin layer chromatography (TLC) and nearly pure by gas chromatography...
Embodiment 2
[0027] Embodiment 2: preparation compound 3
[0028] The C that embodiment 1 makes 9 Unit 21.5 grams (0.1mol, 98%) and compound 121g (0.12mol, 96%) were stirred in a 250ml three-necked flask with 50ml of n-hexane, the temperature was -10°C, nitrogen was blown, and 4.2N butyllithium was slowly added dropwise 36ml (0.15mol) of n-hexane solution, keeping the reaction temperature at about -10°C to -5°C, the dropwise addition was completed in 30 minutes, continued to stir for 1 hour, and tracked and detected by thin layer chromatography (TLC), C 9 The unit has reacted completely, warmed up to room temperature, added 50ml of water and stirred, separated, the aqueous phase was washed twice with 50ml of n-hexane, merged into the organic phase, and the organic phase was washed with 100ml of dilute acid water until neutral. The organic phase was concentrated under reduced pressure in a water bath at 50°C to obtain 45.2 g of a light red oil. The light red oil was subjected to high vacu...
Embodiment 3
[0029] Embodiment 3: preparation compound 4
[0030] The C that embodiment 1 makes 9 Unit 21.5 grams (0.1mol, 98%) and compound 2 21g (0.12mol, 96%) were stirred in a 250ml three-necked flask with 50ml of n-hexane, the temperature was -10°C, nitrogen was blown, and 4.2N butyl was slowly added dropwise. Lithium n-hexane solution 36ml (0.15mol), keep the reaction temperature at about -10°C ~ -5°C, add dropwise in 30 minutes, continue to stir for 1 hour, track and detect with thin layer chromatography (TLC), C 9 The unit has reacted completely, warmed up to room temperature, added 50ml of water and stirred, separated, the aqueous phase was washed twice with 50ml of n-hexane, merged into the organic phase, and the organic phase was washed with 100ml of dilute acid water until neutral. The organic phase was concentrated under reduced pressure in a water bath at 50°C to obtain 45.5 g of a light red oil. The light red oil was subjected to high vacuum (90-100Pa), the oil temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com