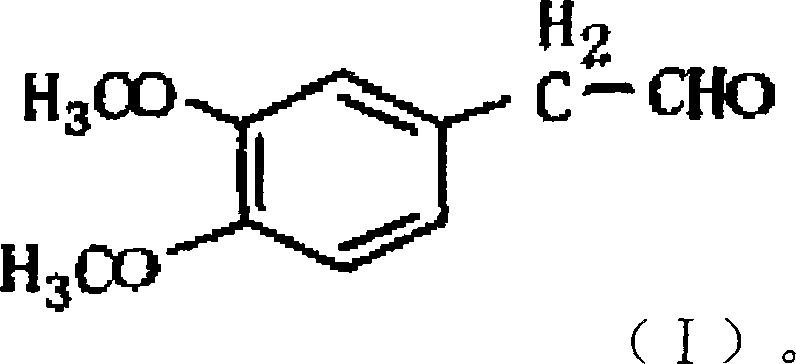
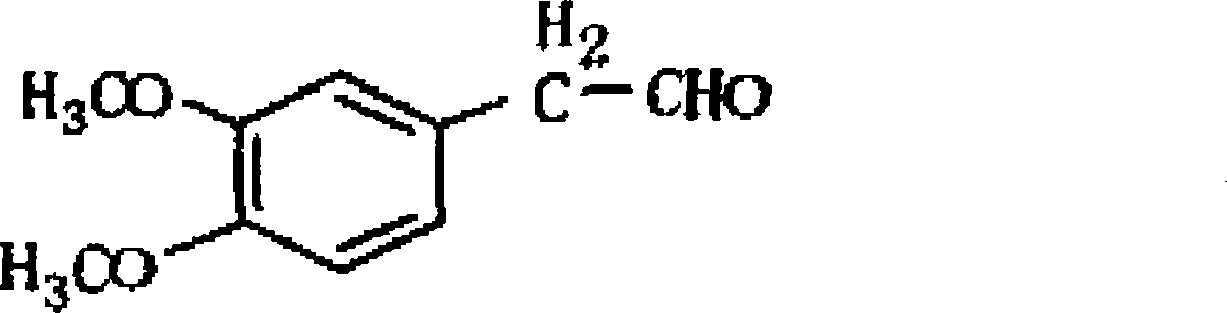A synthesis method of 3,4-dimethoxy hyacinthin
A technology of dimethoxybenzaldehyde and dimethoxybenzene, applied in 3 fields, can solve the problems of difficult industrialized production operation, increased raw material cost, low yield and the like, and is beneficial to large-scale industrialized production and industrial operation. Simple, high product content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] With 3,4-dimethoxybenzaldehyde 8.4g (0.0505mmol), methyl chloroacetate 5.2ml (0.0594mmol), potassium carbonate 8.2g (0.0594mmol), dimethylformamide 25ml, TEBA0.4g (0.0018 mmol), added to the reaction flask, stirred and reacted at room temperature for 48 hours, TLC showed that the raw materials disappeared, added 60ml of water and 30ml of dichloromethane, separated into layers, and the aqueous layer was extracted with 10ml×3 dichloromethane, washed with saturated aqueous sodium chloride solution, anhydrous Dry over magnesium sulfate, filter, drop the filtrate into 7.2g potassium hydroxide and 50ml ethanol solution, precipitate solid, stir and react at room temperature for 4 hours, cool to 0°C, filter to obtain potassium salt.
[0027] Add the solid potassium salt into the mixed solution of water, dichloromethane and potassium dihydrogen phosphate, stir at room temperature for 4 hours, separate layers, extract the water layer with 10ml×3 dichloromethane, wash with saturate...
Embodiment 2
[0029] 16.8g (0.1011mmol) of 3,4-dimethoxybenzaldehyde, 13.3ml (0.1516mmol) of methyl chloroacetate, 20.9g (0.1516mmol) of potassium carbonate, 25ml of tetrahydrofuran, and 0.9g (0.0040mmol) of TEBA were added Reaction bottle, stirred at room temperature for 24 hours, TLC showed that the raw materials disappeared, added 60ml of water and 30ml of toluene, separated, the water layer was extracted with 10ml×3 toluene, washed with saturated aqueous sodium chloride, dried with anhydrous magnesium sulfate, filtered, and the filtrate was dropped Put it into 14.4g potassium hydroxide and 50ml ethanol solution, precipitate solid, stir and react at room temperature for 4 hours, cool to 0°C, filter to obtain potassium salt.
[0030] Add the solid potassium salt into the mixed solution of water, dichloromethane and potassium dihydrogen phosphate, stir at room temperature for 4 hours, separate layers, extract the water layer with 10ml×3 dichloromethane, wash with saturated aqueous sodium ch...
Embodiment 3
[0032] 4.2g (0.0253mmol) of 3,4-dimethoxybenzaldehyde, 2.9ml (0.0328mmol) of methyl chloroacetate, 2.2g (0.0393mmol) of potassium hydroxide, 12.5ml of dimethylformamide, dichloromethane 12.5ml, TBAB 0.4g (0.0012mmol), add to the reaction bottle, 40 ℃, stirring reaction for 72 hours, TLC shows that the raw material disappears, add 60ml of water and 20ml of dichloromethane, separate layers, the water layer is extracted with 10ml×3 dichloromethane , washed with saturated sodium chloride aqueous solution, dried over anhydrous magnesium sulfate, filtered, and the filtrate was dropped into 3.6g potassium hydroxide and 50ml ethanol solution to precipitate a solid, stirred and reacted at room temperature for 4 hours, cooled to 0°C, filtered to obtain potassium salt.
[0033] Add the solid potassium salt into the mixed solution of water, dichloromethane and potassium dihydrogen phosphate, stir at room temperature for 4 hours, separate layers, extract the water layer with 10ml×3 dichloro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com