Method for synthesizing aminoglucose tetrasaccharide
A technology of glucosamine and a synthetic method, applied in the field of preparation of oligosaccharides, can solve problems such as complicated steps, and achieve the effects of simple steps, suitable for mass preparation, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
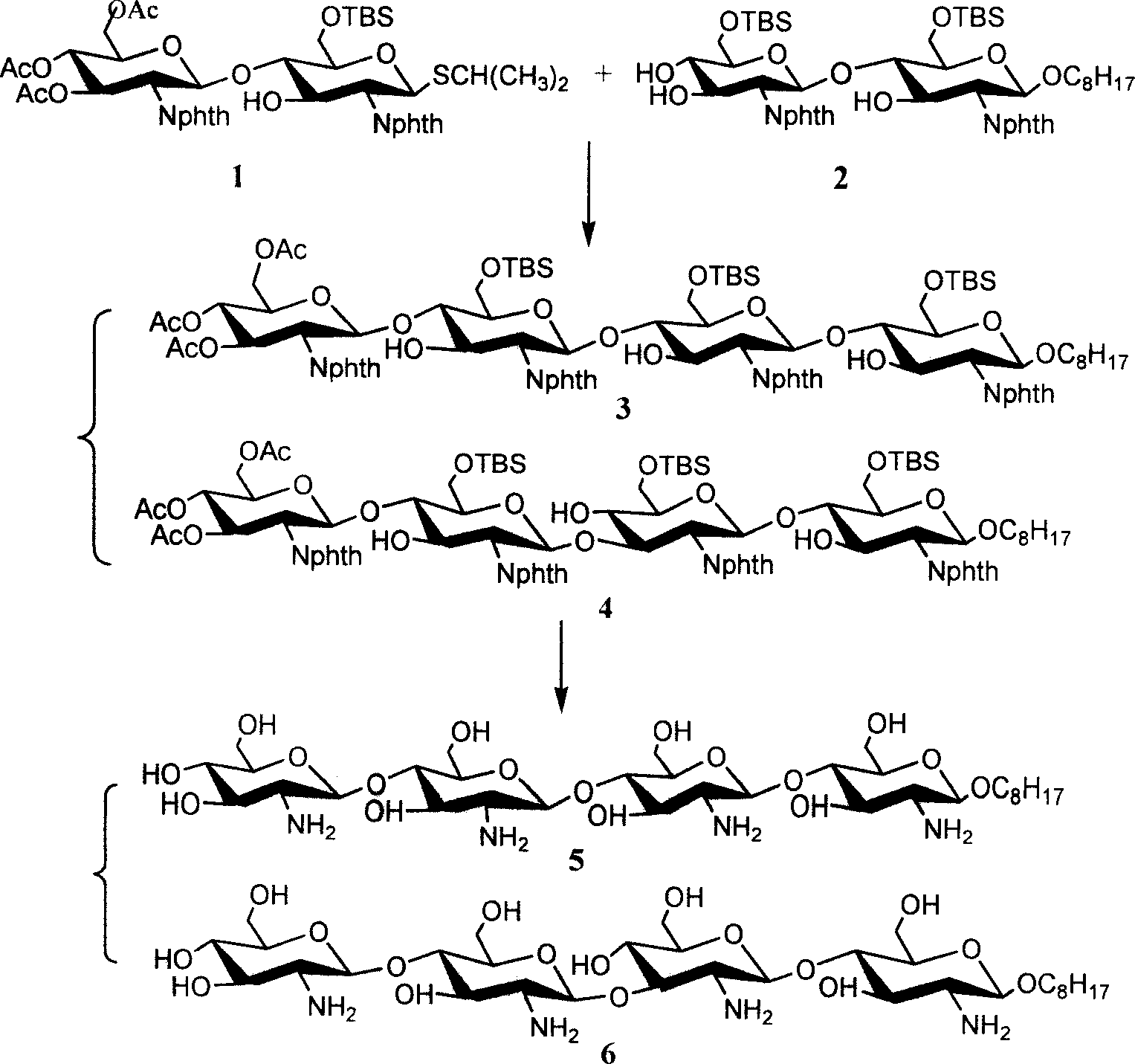
[0032] Below in conjunction with embodiment the present invention is described in detail:
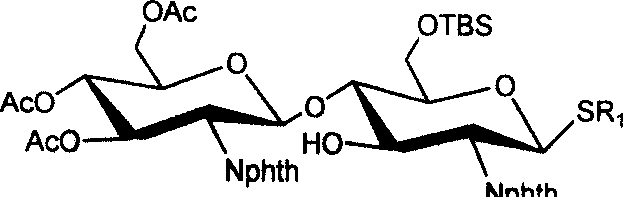
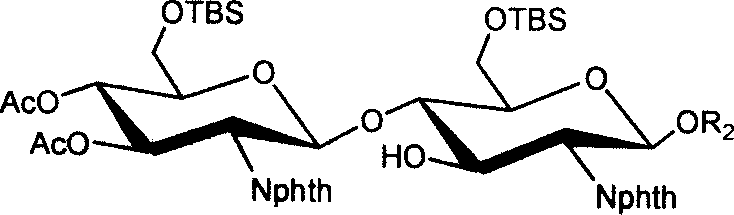
[0033] (1) 3,4,6-tri-oxo-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranose-(1→4)-6-oxo- Synthesis of tert-butyldimethylsilyl-2-deoxy-2-phthalimido-β-D-glucopyranose isopropylthioside 1
[0034] Isopropylthio β-D-glucopyranoside 8 (1.4 g, 2.9 mmol) and 1.1 equivalents of trichloroacetimidate 7 (1.62 g, 2.8 mmol) of the donor compound glucopyranose were dissolved In 15ml of dry dichloromethane, cool to -10°C in an ice-salt bath, in a nitrogen atmosphere, add 0.07 equivalent (35μl) of trimethylsilyl trifluoromethanesulfonate to catalyze the coupling reaction After reacting for half an hour, two drops of triethylamine were added to neutralize the reaction system, the solvent was distilled off under reduced pressure, purified and separated by silica gel chromatography, and 1.34 g of solid foam 1 was obtained. Acetylated 1 1 H NMR (CDCl 3 )δ: 0.08, 0.09(2s, 6H, Si(CH 3 ) 2 ), 0.93(s, 9H, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com