Chirality diamine-metallic complex polyphase catalyzer as well as preparation method and application
A technology of metal complexes and heterogeneous catalysts, applied in the direction of organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, catalyst supports, etc., to achieve high activity, chiral selectivity, high stability, easy to prepare effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1: Preparation of chiral diamine-metal complex heterogeneous catalyst
[0074] 1) Preparation of water-soluble magnetic fluid
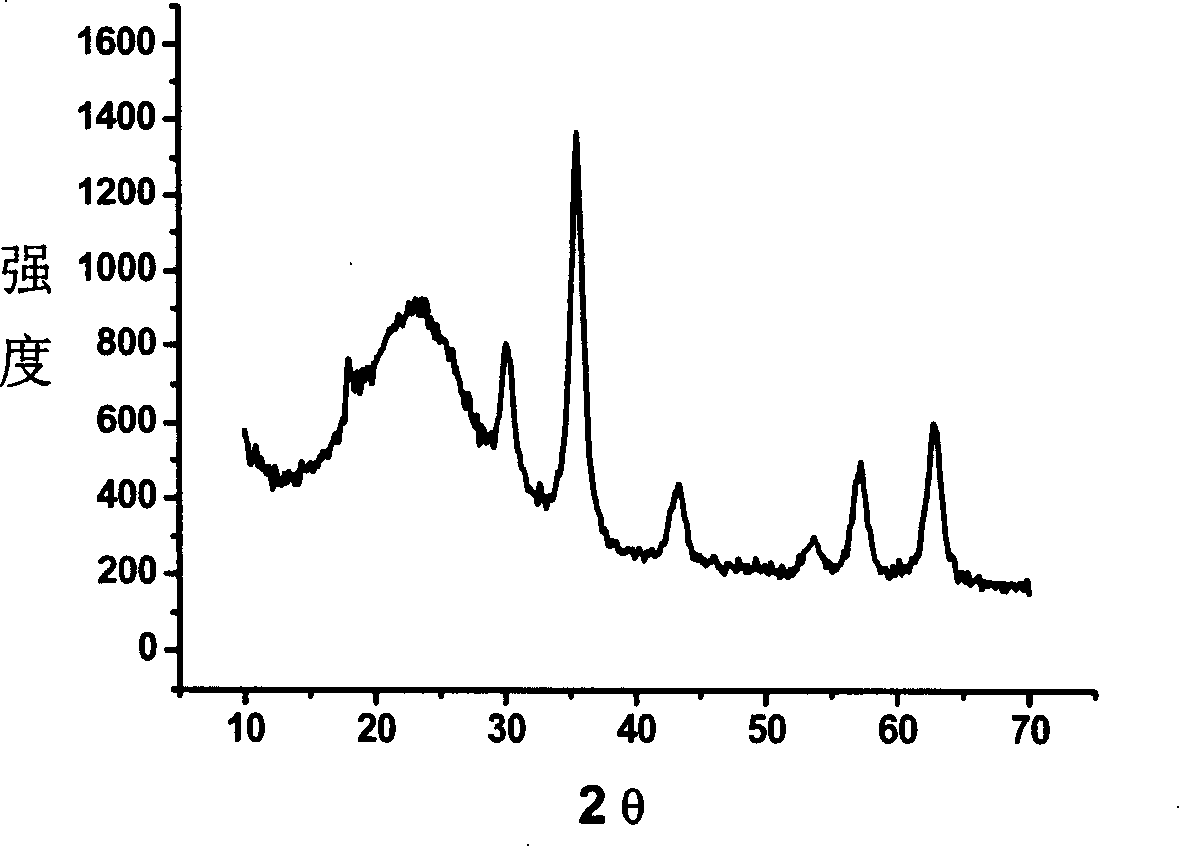
[0075] 1.25g FeCl 3 ·6H 2 O and 0.46FeCl 2 4H 2 O was dissolved in 75ml of degassed deionized water, and the temperature was raised to 80°C under the protection of argon. Under vigorous mechanical stirring, 19ml of 28% ammonia water was quickly injected and kept for 20min. Cool down to room temperature, wash with deionized water to neutrality, add 0.45g dopamine hydrochloride, and ultrasonicate for 30min to obtain a water-soluble magnetic fluid with an average particle size of 10-15nm (see figure 1 ).
[0076] 2) Preparation of oil-soluble magnetic fluid
[0077] Same as step 1. 1.25g FeCl 3 ·6H 2 O and 0.46FeCl 2 .4H 2 O was dissolved in 75ml of degassed deionized water, and the temperature was raised to 80°C under the protection of argon. Under vigorous mechanical stirring, 19ml of 28% ammonia water was quickly injected a...
Embodiment 2
[0083] Example 2: Heterogeneous catalysts for asymmetric hydrogen transfer reactions
[0084]Add 0.5mmol acetophenone, 340mgNaCOOH to the heterogeneous catalyst prepared in embodiment 1 step 6 and react at 40°C. After the reaction, remove the solid catalyst with magnet adsorption (see image 3 ), the filtrate was purified by column chromatography and analyzed by gas chromatography for conversion and enantioselectivity (full methylated β-cyclodextrin chiral capillary column), the results are shown in Table 1.
Embodiment 3
[0085] Embodiment 3: heterogeneous chiral catalyst is used for asymmetric hydrogen transfer reaction of acetophenone
[0086] The heterogeneous catalyst prepared in embodiment 1, add 0.5mmol acetophenone, 0.13ml HCOOH, 0.37ml Et 3 N was reacted at 40°C, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com