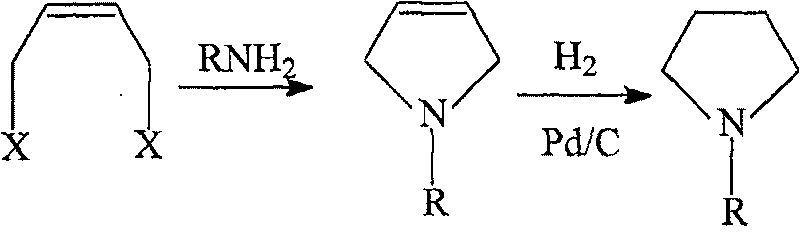
Method for preparing N-alkyl pyrrolidine
A technology of ethylpyrrolidine and methylpyrrolidine, which is applied in the field of pharmaceutical and pesticide intermediates, can solve the problems of harsh reaction conditions and large equipment corrosion, and achieve the effects of low equipment requirements, high product yield and good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Preparation of N-methyl-3-dihydropyrrole
[0028] Add 40% 233.3g (3.0mol) monomethylamine aqueous solution to a 500ml reaction flask equipped with a thermometer, condenser, magnetic stirring and constant pressure dropping funnel, control it at 30°C, and add 125.0g (1.0mol) ) cis-1,4-dichloro-2-butene and 40% NaOH solution, the pH of the system is controlled at 11~12. After the dropwise addition, the temperature was raised to 60° C., the pH of the system was controlled to be 11-12, and the reaction was continued for 3 hours. After the reaction was finished, 63.5 g of N-methyl-3-dihydropyrrole was obtained through two rectifications, with a yield of 76.5%.
[0029] (2) Preparation of N-Methylpyrrolidine
[0030] In a 2L autoclave, add 500g tetrahydrofuran, 83.0g (1.0mol) N-methyl-3-dihydropyrrole and 4.2g 5% Pd / C catalyst (the mass percentage of Pd is 5%), and the temperature is raised to 60°C , start to pass H after purging the air with nitrogen 2 , control the p...
Embodiment 2
[0032] (1) Preparation of N-ethyl-3-dihydropyrrole
[0033] Add 50% 180.0g (2.0mol) monoethylamine aqueous solution to a 500ml reaction flask equipped with a thermometer, condenser tube, magnetic stirring and constant pressure dropping funnel, control it at 30°C, and drop 214.0g (1.0mol) ) cis-1,4-dibromo-2-butene and saturated Na 2 CO 3 solution, the pH of the system is controlled at 11-12. After the dropwise addition, the temperature was raised to 100° C., the pH of the system was controlled to be 11-12, and the reaction was continued for 1 h. After the reaction was finished, 58.5 g of N-ethyl-3-dihydropyrrole was obtained through two rectifications, with a yield of 60.3%.
[0034] (2) Preparation of N-ethylpyrrolidine
[0035] Add 97g of methanol, 97.0g (1.0mol) of N-ethyl-3-dihydropyrrole and 1.0g of 5% Pd / C catalyst into a 2L autoclave, raise the temperature to 100°C, and start to pass H after purging the air with nitrogen 2 , control the pressure of the reaction sys...
Embodiment 3
[0037] (1) Preparation of 3-dihydropyrrole
[0038] Add 10% 680.0g (4.0mol) of ammonia water to a 1000ml reaction flask equipped with a thermometer, magnetic stirring and constant pressure dropping funnel, control at 30°C, and dropwise add 125.0g (1.0mol) of cis-1,4 -Dichloro-2-butene. After the dropwise addition, the temperature was raised to 30° C., and the reaction was continued for 8 hours. After the reaction, 37.4 g of 3-dihydropyrrole was obtained through two rectifications, with a yield of 54.2%.
[0039] (2) Preparation of pyrrolidine
[0040] In a 2L autoclave, add 621g of isopropanol, 69.0g (1.0mol) of 3-dihydropyrrole and 3.5g of 10% Pd / C catalyst (the mass percent of Pd is 10%), heat up to 30°C, and purge with nitrogen Start to pass H after the air 2 , control the pressure of the reaction system to 1.0 MPa, stir the reaction for 2 hours, and cool down to room temperature after the reaction. The Pd / C catalyst was recovered by filtration, and the liquid was rect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com