Human body hard tissue filling renovation material containing calcium citrate
A technology of calcium citrate and repair materials, which is applied in the fields of prosthesis, medical science, organic chemistry, etc. It can solve the problems of affecting the formation of new bone tissue, difficult biodegradation or absorption, application restrictions, etc., and achieve good bone conduction and repair effects , good biocompatibility, and wide application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Prepare tricalcium citrate according to the literature method (Wang Shanyang, Weng Zhenhui, He Yanhong. Prepare calcium citrate from shells [J]. Journal of Hangzhou Normal University, 1995 (6): 69-73), or pharmaceutical grades available on the market Tricalcium citrate is the main raw material (100%), which is granulated, dried, and prepared into lumps with a tablet press under a pressure of 6 mPA at normal temperature.
[0020] The use effect verification experiment is as follows:
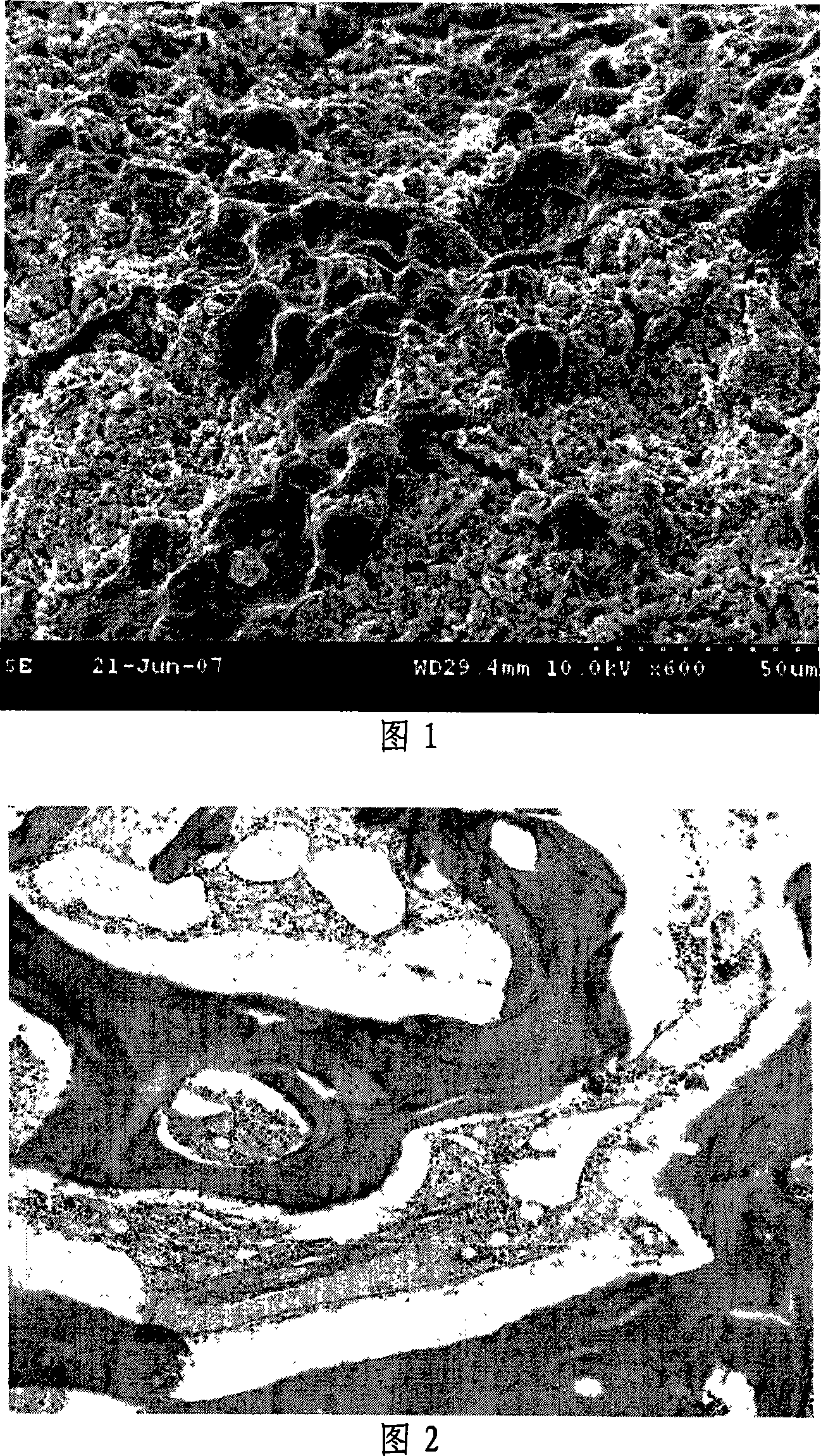
[0021] cobalt 60 After disinfection, it was co-incubated with rabbit osteoblasts in vitro, cell proliferation and differentiation were observed at 7 days, and the relative cell proliferation rate (RGR) was calculated. Calculation formula: RGR=(test group X / negative control group X)×100%, according to the 6-level toxicity scoring standard, the material toxicity score is 1, without any cytotoxicity, and cells can adhere and proliferate on the surface of tricalcium citrate ( As shown in Figu...
Embodiment 2
[0023] Adopting tricalcium citrate as the main material (85%), after mixing with auxiliary materials fibrin glue (14.5%) and tetracycline (0.5%), through granulation, drying, under normal temperature, under the pressure of 10mPA, a tablet press is prepared into Blocks or flakes are prepared and shaped.
[0024] The use effect verification experiment is as follows:
[0025] cobalt 60 After disinfection, it was co-incubated with rabbit osteoblasts in vitro, cell proliferation and differentiation were observed at 7 days, and the relative cell proliferation rate (RGR) was calculated. Calculation formula: RGR=(test group X / negative control group X)×100%, according to the 6-level toxicity scoring standard, the material toxicity score is level 1, without any cytotoxicity. The material was implanted into the back muscle bag of rats in vivo, and histology confirmed that the biomaterial had no adverse reactions and had good biocompatibility; it was degradable and drug-sustained-releas...
Embodiment 3
[0027] Tricalcium citrate is used as the main material (65%), mixed with auxiliary materials polyvinylpyrrolidone (34.5%) and vancomycin (0.5%); after granulation, drying, and compression at room temperature under a pressure of 5mPA Machine prepared into blocks.
[0028] The use effect verification experiment is as follows:
[0029] cobalt 60 After disinfection, it was co-incubated with rabbit osteoblasts in vitro, cell proliferation and differentiation were observed at 7 days, and the relative cell proliferation rate (RGR) was calculated. Calculation formula: RGR=(test group X / negative control group X)×100%, according to the 6-level toxicity scoring standard, the material toxicity score is level 1, without any cytotoxicity. The material was implanted into the back muscle bag of rats in vivo, and histology confirmed that the biomaterial had no adverse reactions and had good biocompatibility; it was degradable and drug-sustained-release activity (degradation time in vivo was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com