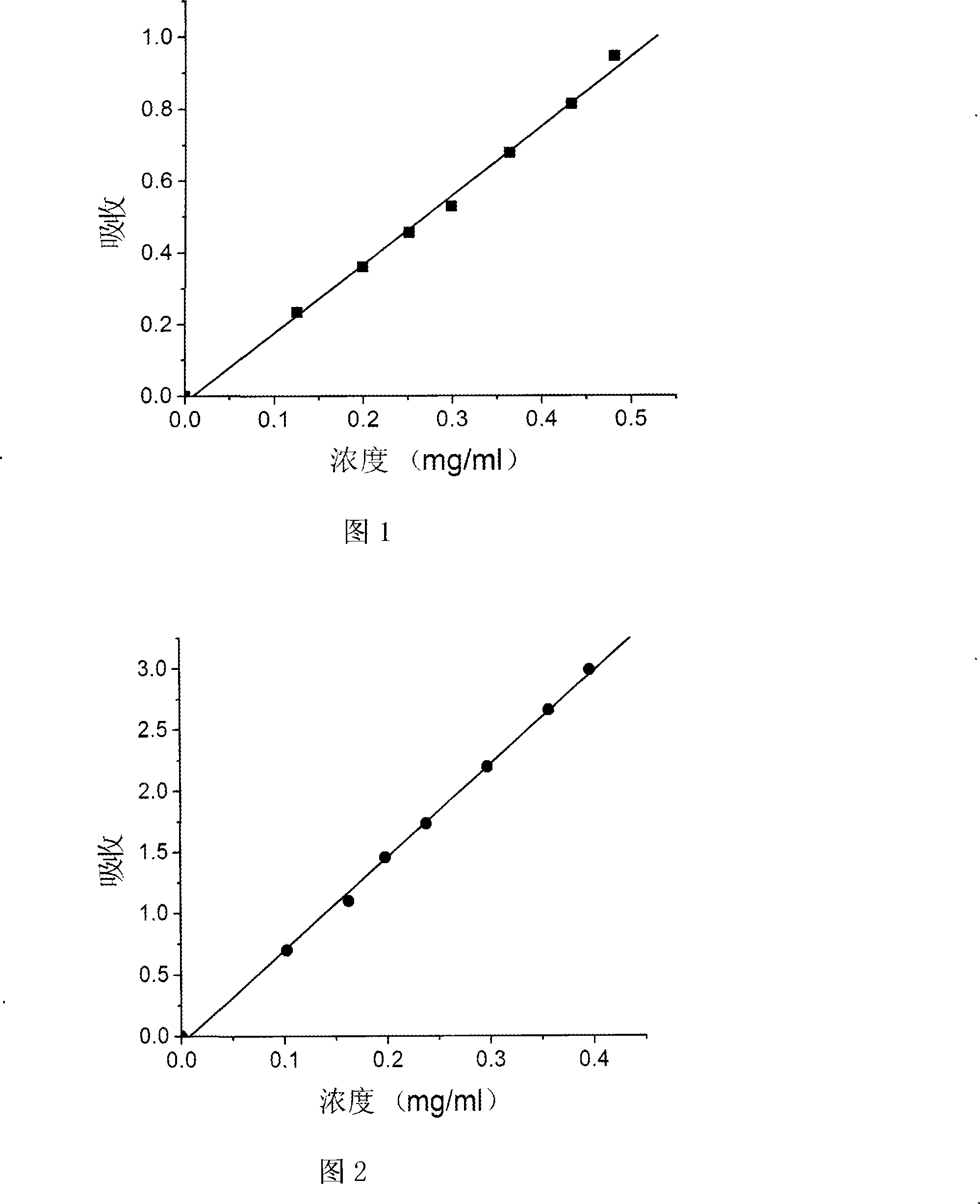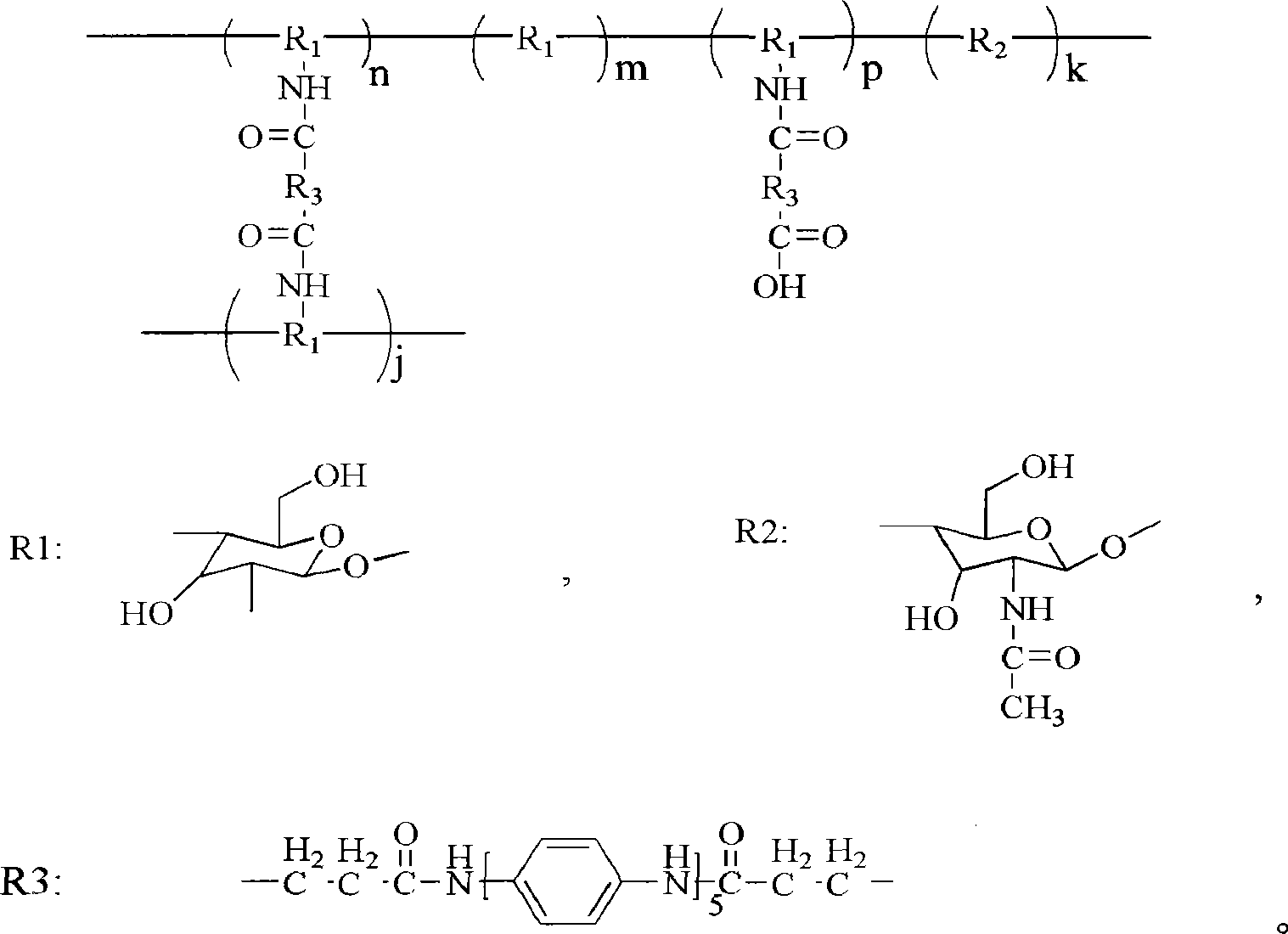Method for preparing dicarboxylanilin pentamer cross-linking chitosan polymer
A technology of aniline pentamer and cross-linked chitosan, which is applied in the field of biomedical polymer materials, can solve the problems of poor compatibility, non-degradable solubility, difficult processing, etc., and achieve good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
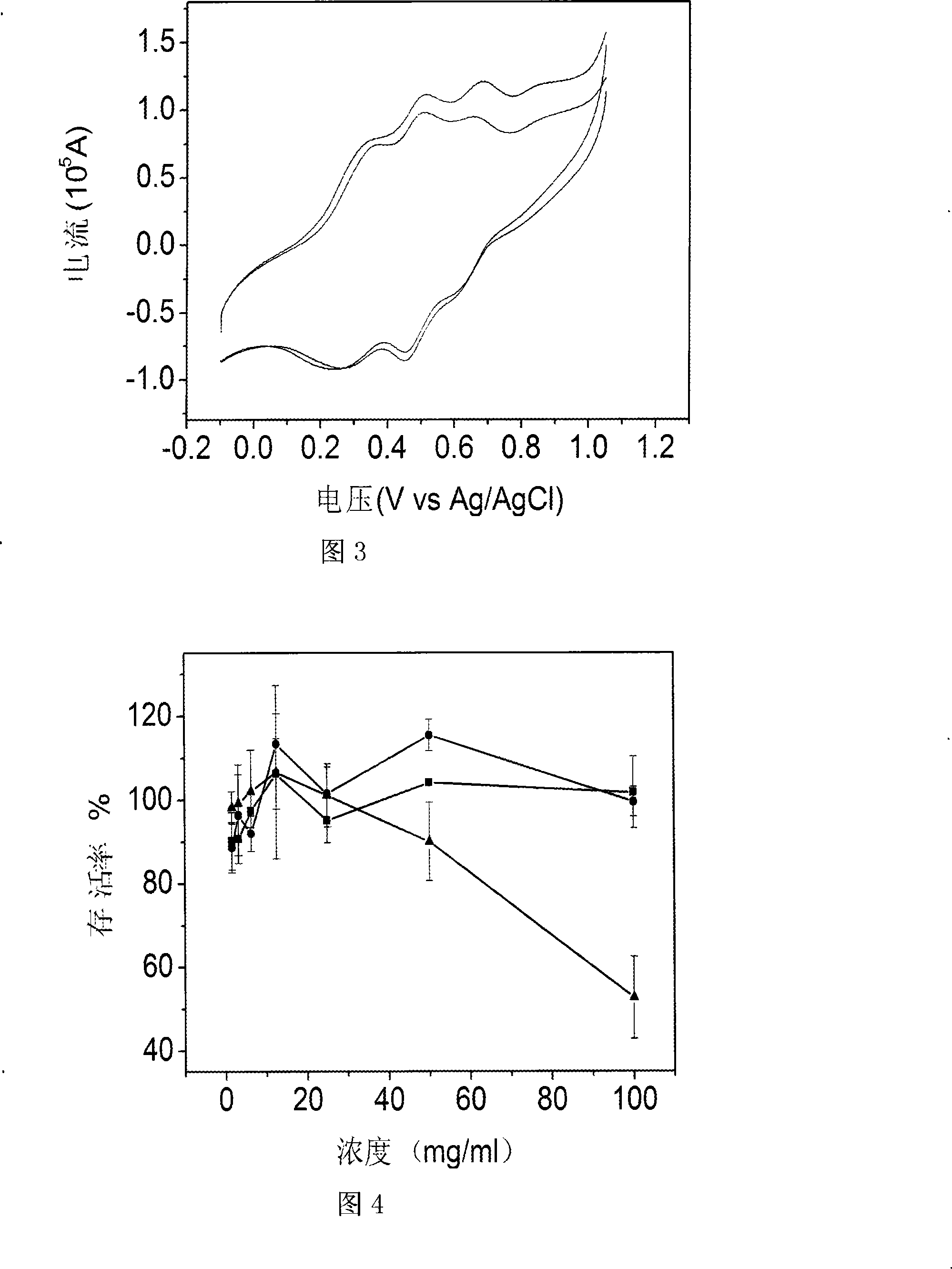
[0034] Embodiment 1: the terminal carboxyl group of activation aniline pentamer
[0035] Under anhydrous and oxygen-free conditions, dissolve 1.5g (0.00223mol) of dicarboxyl-terminated aniline pentamer, 0.642g (0.00446mol) NHS and 0.92g (0.00446mol) DCC in 20ml DMF, stir at room temperature for 24h, and filter , the reaction product was precipitated with 400ml of cold ethanol, filtered with suction, and vacuum-dried at 45°C for 48h to obtain 1.597g of aniline pentamer activated by carboxyl groups at both ends. Yield 93%.
Embodiment 2
[0036] Embodiment 2: the synthesis of aniline pentamer cross-linked chitosan, wherein the content of aniline pentamer is 2.4%
[0037] Get commercial chitosan (molecular weight 40,000, degree of deacetylation is 75%) 0.45g, be dissolved in 30ml acetic acid concentration under the condition of stirring and be in the 10v% acetic acid aqueous solution, at room temperature and under the condition of stirring, by dropping funnel Add 20ml of DMSO dropwise into the aqueous acetic acid solution at a rate of 1 drop every 3-5 seconds. 0.05 g of the aniline pentamer with activated carboxyl groups at both ends obtained in Example 1 was dissolved in 10 ml of DMF and placed in a constant pressure dropping funnel. Under the protection of nitrogen, the temperature of the reaction system is raised to 50°C by means of air extraction technology, and the DMF solution is added dropwise to the mixed solution of acetic acid aqueous solution and DMSO at a rate of 1 drop every 3-5 seconds. Then react...
Embodiment 3
[0038] Embodiment 3: the synthesis of aniline pentamer cross-linked chitosan, wherein the content of aniline pentamer is 4.9%
[0039] Get commercial chitosan (molecular weight 40,000, degree of deacetylation is 75%) 0.8g, be dissolved in 30ml acetic acid concentration under the condition of stirring and be in the 10v% acetic acid aqueous solution, at room temperature and under the condition of stirring, by dropping funnel Add 20ml of DMSO dropwise into the aqueous acetic acid solution at a rate of 1 drop every 3-5 seconds. 0.2 g of the aniline pentamer obtained in Example 1 with carboxyl groups at both ends activated was dissolved in 10 ml of DMF and placed in a constant pressure dropping funnel. Under the protection of nitrogen, the temperature of the reaction system is raised to 50°C by means of air extraction technology, and the DMF solution is added dropwise to the mixed solution of acetic acid aqueous solution and DMSO at a rate of 1 drop every 3-5 seconds. Then react a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com