Application of cholinesterase in antagonistic tachykinin medicine
A technology of cholinesterase and tachykinin, which is applied in the application field of medicine to achieve the effects of small side effects, suitable for industrialization, and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
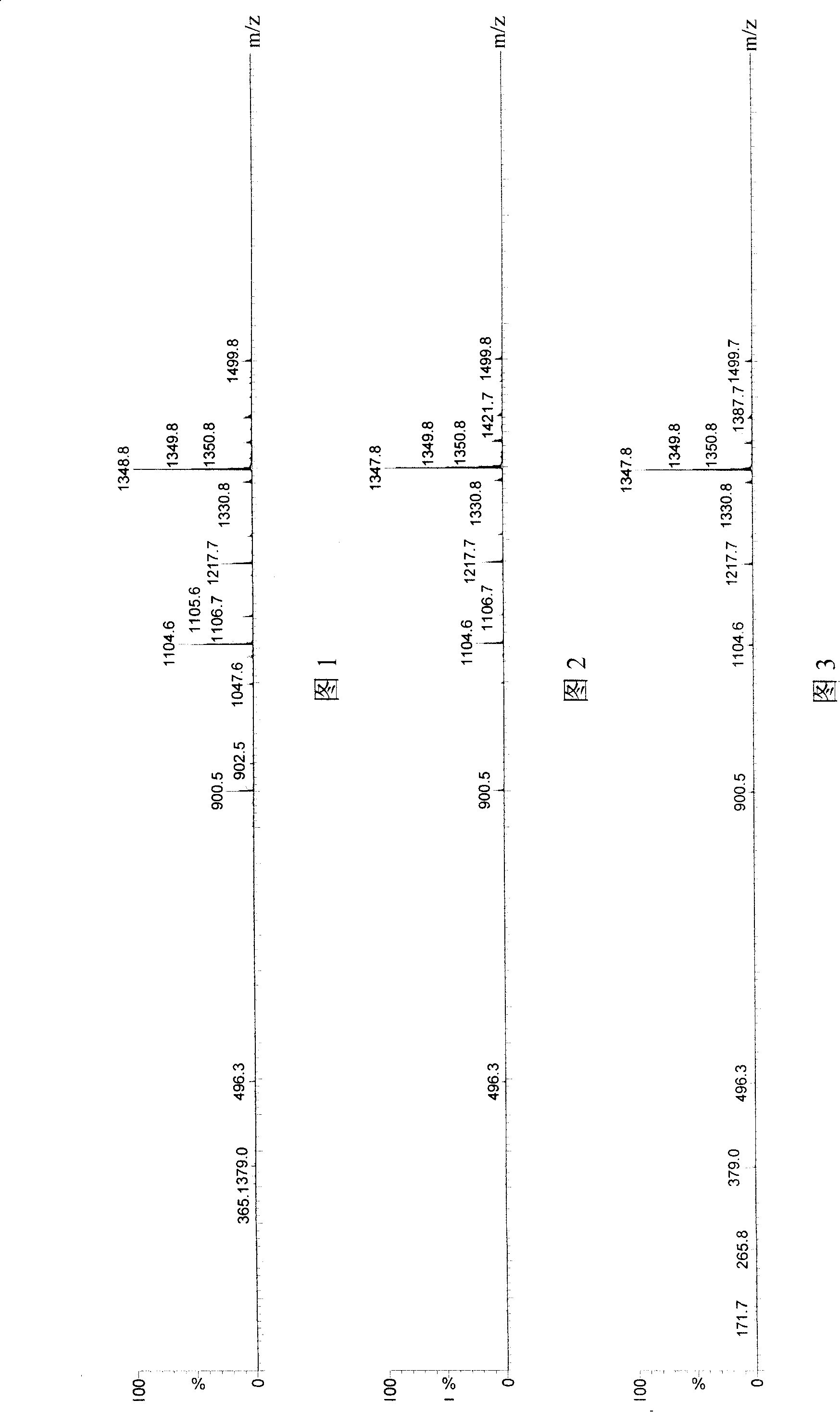
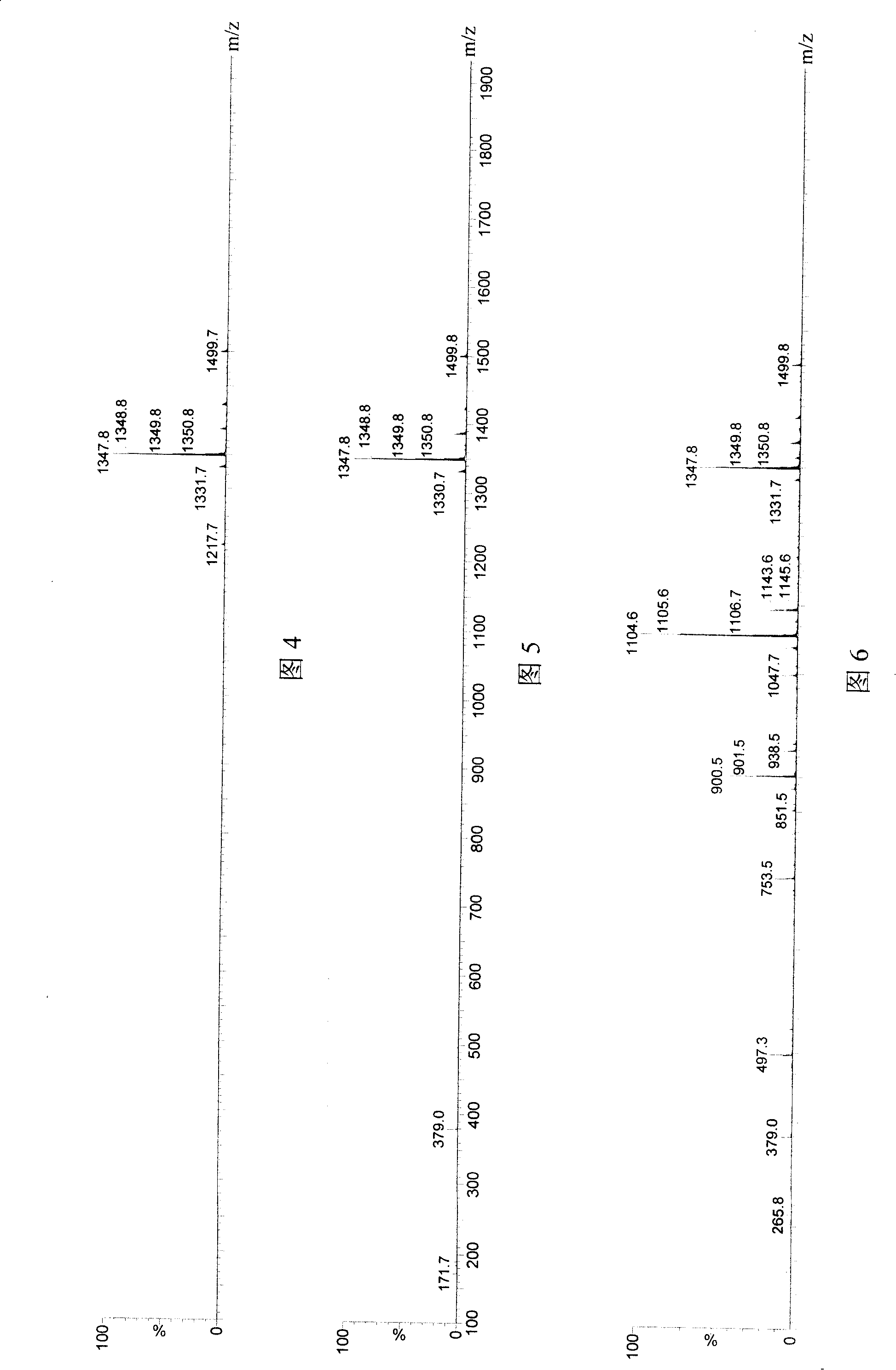
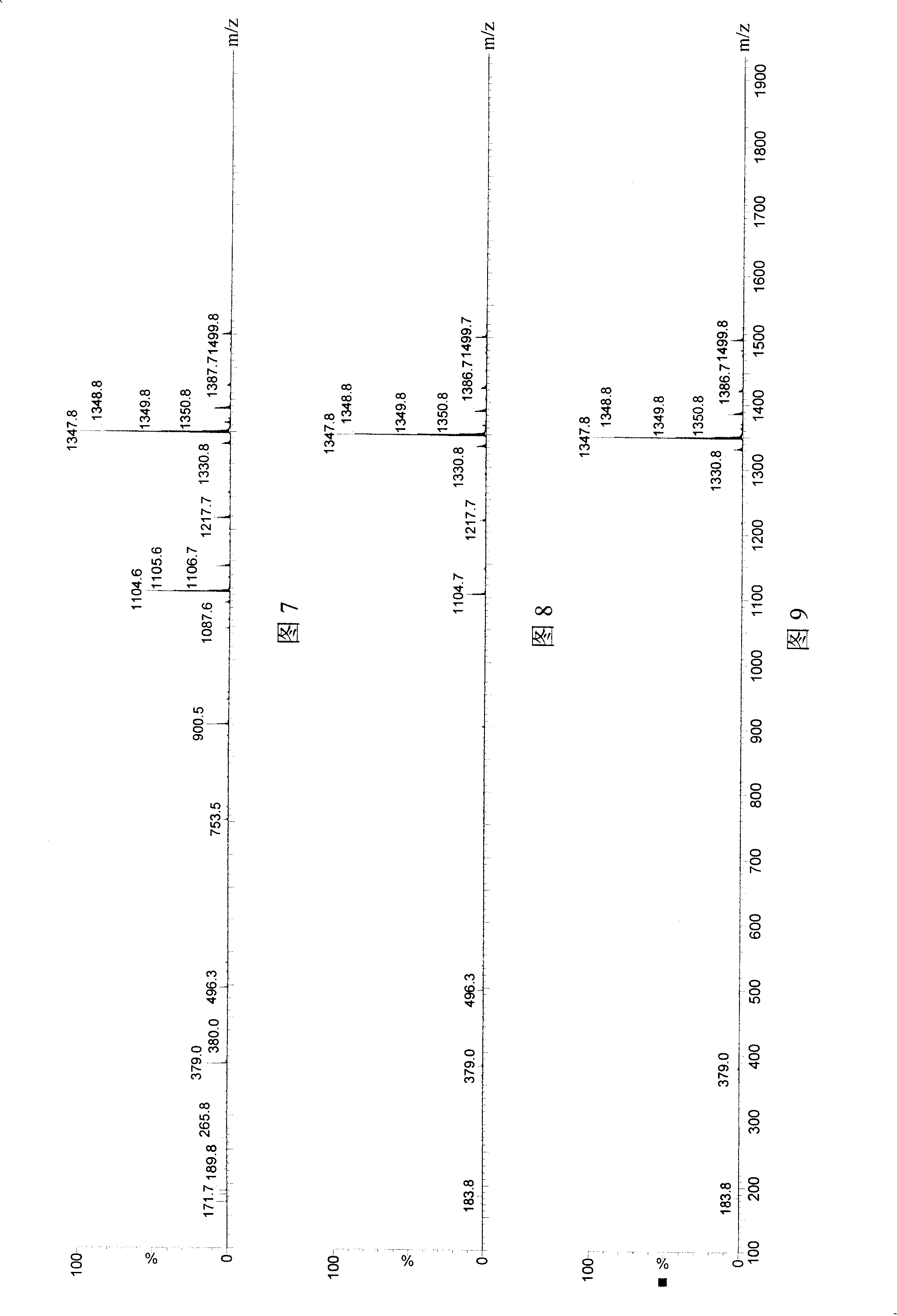
[0070] Embodiment 1 hydrolysis SP test
[0071] Butyrylcholinesterase and acetylcholinesterase, which are used in the market as an enzyme inhibition method to detect pesticide residues, were used to hydrolyze substance P respectively. Reaction and detection conditions:
[0072] 1. Cholinesterase used:
[0073] (1) butyrylcholinesterase, specific activity 200U freeze-dried powder 50mg with 5ml iron ion water (F 3+ Content 1mg / L) dissolved, centrifuged at 10,000 rpm for 4 minutes;
[0074] (2) butyrylcholinesterase, specific activity 200U lyophilized powder 50mg dissolved in 5ml double distilled water, centrifuged at 10000 rpm for 4 minutes;
[0075] (3) Acetylcholinesterase, specific activity 300U freeze-dried powder 20mg dissolved in 1ml double distilled water, centrifuged at 10000 rpm for 4 minutes.
[0076] 2. Substrate
[0077] The purity of SP is 98%, and the concentration of the aqueous solution is 0.5ug / ml.
[0078] 3. Response
[0079] Substrate+enzyme 100ul+100u...
Embodiment 2
[0086] Embodiment 2 prepares acetylcholinesterase aqueous solution
[0087] Use acetylcholinesterase freeze-dried powder produced by SIGMA company, add water to dissolve, add stabilizer propylene glycol, antibacterial agent phenol at the same time, according to each milliliter solution contains acetylcholinesterase enzyme activity from 10 to 1000U, divide into several orders of magnitude, according to the way of use, pack them separately Disposable syringes, spray bottles, or other medically acceptable vials, kept refrigerated. The storage period can be up to 1 year, and it can be administered directly or diluted according to the treatment requirements.
[0089] 1. Theoretical basis: According to the Ellman method, thioacetylcholine iodide (BTCI) is hydrolyzed into thiocholine and acetic acid under the action of cholinesterase (AChE), and thiocholine can be combined with dithiop-nitrobenzoic acid (DTNB) reacts to generate a yellow product, which has ...
Embodiment 3
[0092] Embodiment 3 aqueous solution 2
[0093] Aqueous solution 1 is added with 5% propylene glycol and 0.5% laurocaprazine as a penetration enhancer, combined with transdermal absorption methods such as iontophoresis or ultrasonic method, for external use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com