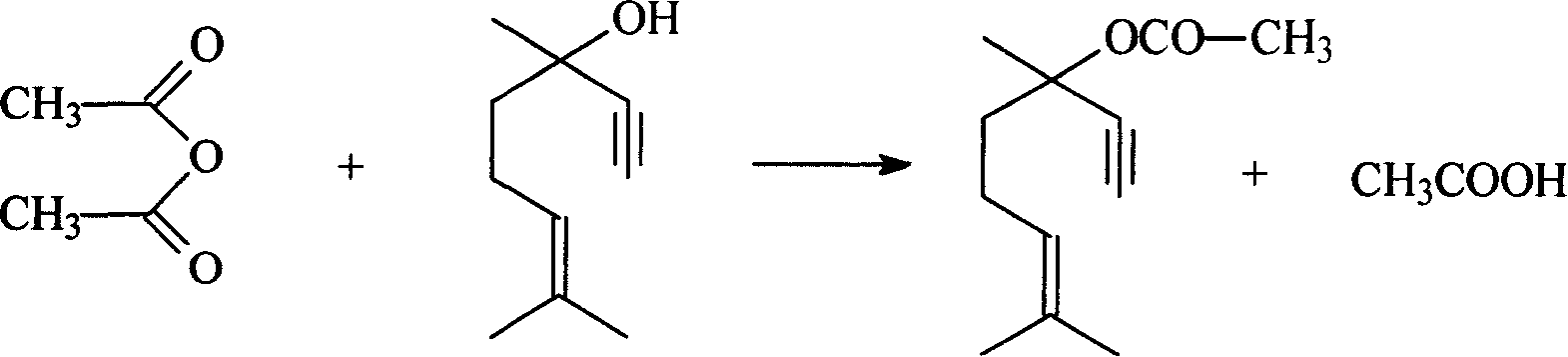
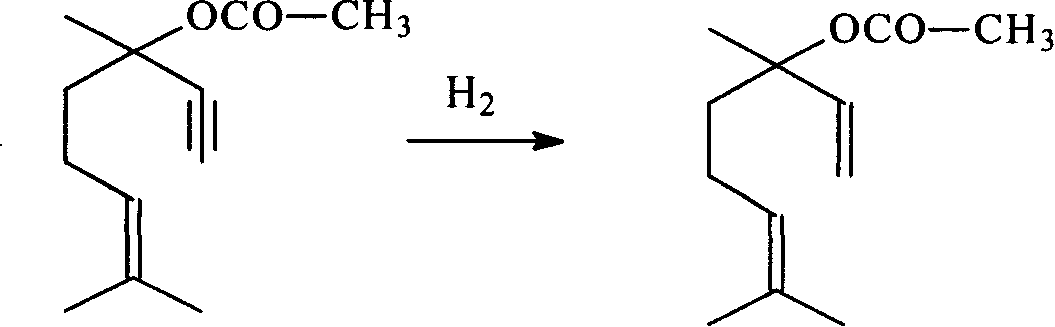
Method for preparing linalyl acetate from dehydrolinalool
A technology of dehydrolinalool and linalyl acetate, which is applied to the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, and can solve the problems of difficult to manufacture high-grade fragrances, long time required for esterification reaction, Difficulty in refining hydrogenation products, etc., to achieve ideal conversion rate and selectivity, high quality, and convenient removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~11
[0036] A 500ml three-necked flask with a stirring device is placed in a water bath, 152g of dehydrolinalool is added, nitrogen is purged for 20min, the temperature is raised to the reaction temperature with stirring, and acetic anhydride is dropped in according to the required molar ratio, and the The required amount of catalyst sulfosalicylic acid starts the reaction and is maintained for the required reaction time to complete the reaction. The product is cooled, washed with water, and statically layered to separate the oil and water phases until the oil phase material is neutral, and the oil phase material is taken to obtain the esterification product dehydrolinalyl acetate.
[0037] The specific esterification reaction conditions of Examples 1-11 are shown in Table 1, and the esterification reaction results are shown in Table 2.
[0038] Table 1.
[0039] temperature reflex
[0040] Note: 1) catalyst consumption is the weight percent of raw material feeding to...
Embodiment 12~23
[0046] Preparation of hydrogenation catalyst:
[0047] The starting materials of each active component are: palladium nitrate solution is used for Pd; lead nitrate solution is used for Pb; bismuth nitrate solution is used for Bi in component A, and zinc nitrate solution is used for Zn.
[0048] The above-mentioned starting materials are prepared into an impregnation solution according to the required ratio; a certain amount of calcium carbonate carrier is placed in the impregnation solution and impregnated to obtain a catalyst precursor. The amount of the impregnation solution is 95% to 110% of the water absorption value of the carrier. and 70°C for 1 hour; the catalyst precursor was dried and then roasted at 280°C for 5 hours; the roasted catalyst precursor was reduced with hydrazine hydrate; it was washed several times until the pH value in the washing liquid was close to Neutral, and finally dried at 75°C to obtain the finished catalyst for future use.
[0049] The active ...
Embodiment 24~31
[0058] Using the hydrogenation catalyst of Example 19, the hydrogenation reaction conditions were changed. The changed reaction conditions are shown in Table 5, and the rest are the same as in Examples 12-23. The results are shown in Table 6.
[0059] table 5.
[0060] temperature reflex
(℃)
hydrogen pressure
(Mpa)
Reaction time
(min)
Catalyst accounts for the reaction solution
(wt%)
Example 24
30
1.5
100
1.0
Example 25
20
3.0
240
0.2
Example 26
50
2.5
110
0.2
Example 27
80
2.5
60
5.0
Example 28
50
3.5
120
0.01
Example 29
90
5.5
90
2.0
Example 30
65
2.0
180
0.5
Example 31
150
10
30
0.1
[0061] Table 6.
[0062] Conversion rates
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com