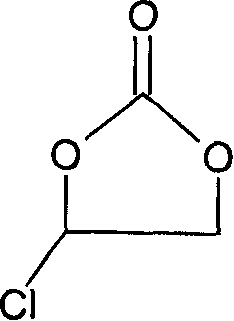
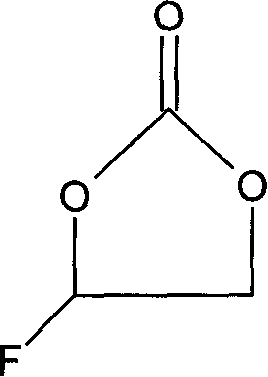
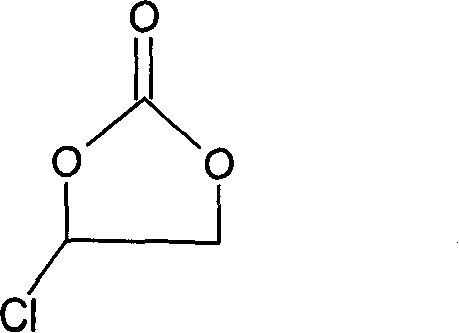
Method for preparing fluoroethylene carbonate
A technology of fluoroethylene carbonate and chloroethylene carbonate, applied in the field of ethylene carbonate derivatives, can solve the problems of highly toxic fluorine gas, high reactivity, long reaction time, etc., and achieves lower production cost and environmental friendliness. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0024] Add 44g sodium fluoride and stir in the three-neck round-bottomed flask equipped with dropping funnel, condenser, stirrer and thermometer, add 3.4g polyethylene glycol phase transfer catalyst (polymerization degree 800), then dropwise add 122.5g chlorine Substitute ethylene carbonate, and control the dropping temperature at 50-70°C. As the dropping goes on, the color of the mixture turns dark brown, and the temperature rises by itself. After the dropping is completed, the mixture is very viscous. After insulated at 100°C for 3 hours, cool the material to 30°C, and then use centrifugal suction to obtain a black filtrate, which contains 0.5% unreacted chloroethylene carbonate, 92% fluoroethylene carbonate and other raw materials with incoming impurities. The black filtrate was subjected to rectification under reduced pressure to finally obtain 59 g of a product with a purity of 99.9%.
[0025] Through the comparison of this example and Comparative Example 1, it can be fo...
Embodiment 3
[0027] In this embodiment, the order of addition of the materials in the second embodiment is reversed. That is: first add 2.8g of polyethylene glycol phase transfer catalyst (polymerization degree 800) and 122.5g of chloroethylene carbonate and mix, and then gradually add 42g of sodium fluoride powder into the above mixture through a funnel at 40°C , the addition speed should be slow, control the reaction temperature at 50-70°C, and finish adding in 2 hours. The other steps are the same as in Example 2. After 3 hours of heat preservation, the material becomes extremely viscous, cool the material to 30°C, and then use After centrifugal suction filtration, a black filtrate was obtained, which contained 4% unreacted chloroethylene carbonate, 89% fluoroethylene carbonate and impurities brought in by other raw materials. The black filtrate was subjected to rectification under reduced pressure to finally obtain 41 g of a product with a purity of 99.5%.
[0028] By comparing this e...
Embodiment 4
[0030] In this embodiment, add 42g sodium fluoride and 1.7g crown 18 ether when equipped with a dropping funnel, a three-necked round bottom flask with a condenser, agitator and thermometer, and then add 122.5g chloroethylene carbonate dropwise, The product has exothermic phenomenon, control the dropping temperature at 50-70°C, and then keep the reaction at 100°C for 3 hours. Suction filtration is carried out after the heat preservation is finished, and the filtrate is taken for chromatographic analysis. The content of chloroethylene carbonate is 1%, 91% of fluoroethylene carbonate, and the rest are unidentified impurities brought in the raw materials. Then the filtrate is decompressed and purified. Distillation finally obtained the finished product 57g with a purity of 99.8%.
[0031] Through this example, it can be found that the effect of using the polyethylene glycol phase transfer catalyst is the same as that of the crown ether transfer catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com