Method for preparing annular phosphonate or annular phosphate flame retardant
A cyclic phosphate and cyclic phosphonate technology, which is applied in the manufacture of fire-retardant and flame-retardant filaments, chemical instruments and methods, compounds of elements of Group 5/15 of the periodic table, etc. The problems of volatilization of phosphorus chloride and high acid value of the final product can achieve the effect of simple operation, low acid value and good viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
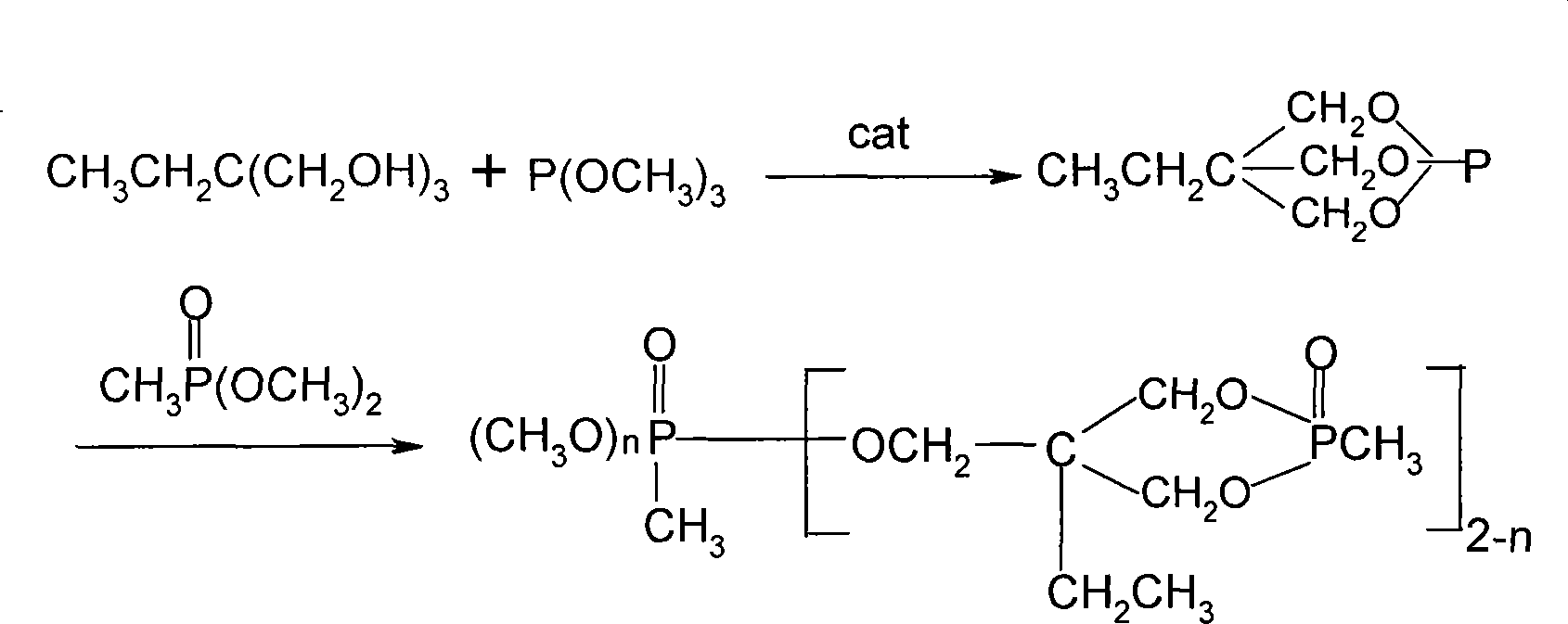
[0020] Example 1 In a four-necked flask equipped with a stirrer, an atmospheric distillation unit, a thermometer, and an air duct, add 62.5g of trimethylolpropane, 63g of trimethyl phosphite, and 1.8g of triethylamine, heat and pass Nitrogen, the temperature was slowly raised to 80°C, and the reaction was carried out at this temperature for 8 hours to obtain the intermediate and methanol, and the methanol was distilled off. Then add 92.5 g of dimethyl methylsulfate to the intermediate, react at 170-190°C for 26 hours, and remove excess dimethylmethylsulfate (applied mechanically) under reduced pressure to obtain a colorless, transparent, highly viscous Liquid 127.5g, yield 95.7%.
Embodiment 2
[0021] Example 2 In a four-necked flask equipped with a stirrer, an atmospheric distillation unit, a thermometer, and an air duct, add 62.5g of trimethylolpropane, 63g of trimethyl phosphite, and 1.8g of triethylamine, heat and pass Nitrogen, slowly warming up to 80 ° C, at this temperature for 6 hours to obtain intermediates and methanol, the methanol was distilled off. Then add 92.5 g of dimethyl methylsulfate to the intermediate, react at 160-180°C for 22 hours, remove excess dimethylmethylsulfate (applied) under reduced pressure, and obtain a colorless, transparent, highly viscous Liquid 123.0g, yield 92.3%.
Embodiment 3
[0022] Example 3 In a four-necked flask equipped with a stirrer, an atmospheric distillation unit, a thermometer, and an air duct, add 62.5g of trimethylolpropane, 63g of trimethyl phosphite, and 1.8g of diethylamine, heat and pass Nitrogen, slowly warming up to 60 ° C, at this temperature for 8 hours to obtain intermediates and methanol, the methanol was distilled off. Then add 92.5 g of dimethyl methylsulfate to the intermediate, react at 165-185°C for 26 hours, and remove the excess dimethylmethylsulfate (applicable) under reduced pressure to obtain a colorless, transparent, highly viscous Liquid 120.0g, yield 90.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com