Novel alpha-amylase inhibitor alcaftadine series compounds and application thereof
A technology of amylase inhibitor and alcatadine, which is applied to a new class of alpha-amylase inhibitor alcatadine series compounds and their application fields, can solve the problems of hydrolysis inactivation development and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Separation and preparation of alcaftadine series compounds
[0035] Cultivate fermented seeds in a 500mL triangular flask, each bottle contains 100mL of liquid medium, which contains: oatmeal 2%, potassium nitrate 0.1%, sodium chloride 0.05%, dipotassium hydrogen phosphate 0.05%, magnesium sulfate 0.05%, ferrous sulfate 0.001 %. Adjust the pH to 7.2-7.5 with NaOH, sterilize with high-pressure steam at 121°C for 20 minutes, and inoculate the slant spores of the strain ZG0656 after cooling [that is, Streptomyces coelicoflavus var.nankaiensis ZG0656 (Streptomyces coelicoflavus var. Preservation Management Committee General Microbiology Center" (No. 13, Zhongguancun North Yiyi, Haidian District, Beijing, Institute of Microbiology, Chinese Academy of Sciences), its preservation number is CGMCC No.2097], cultivated in an air bath shaker at 200rpm 28°C for 48 hours as fermented seeds . Put 7.5L medium of the same composition into a 10L stainless steel mechanica...
Embodiment 2
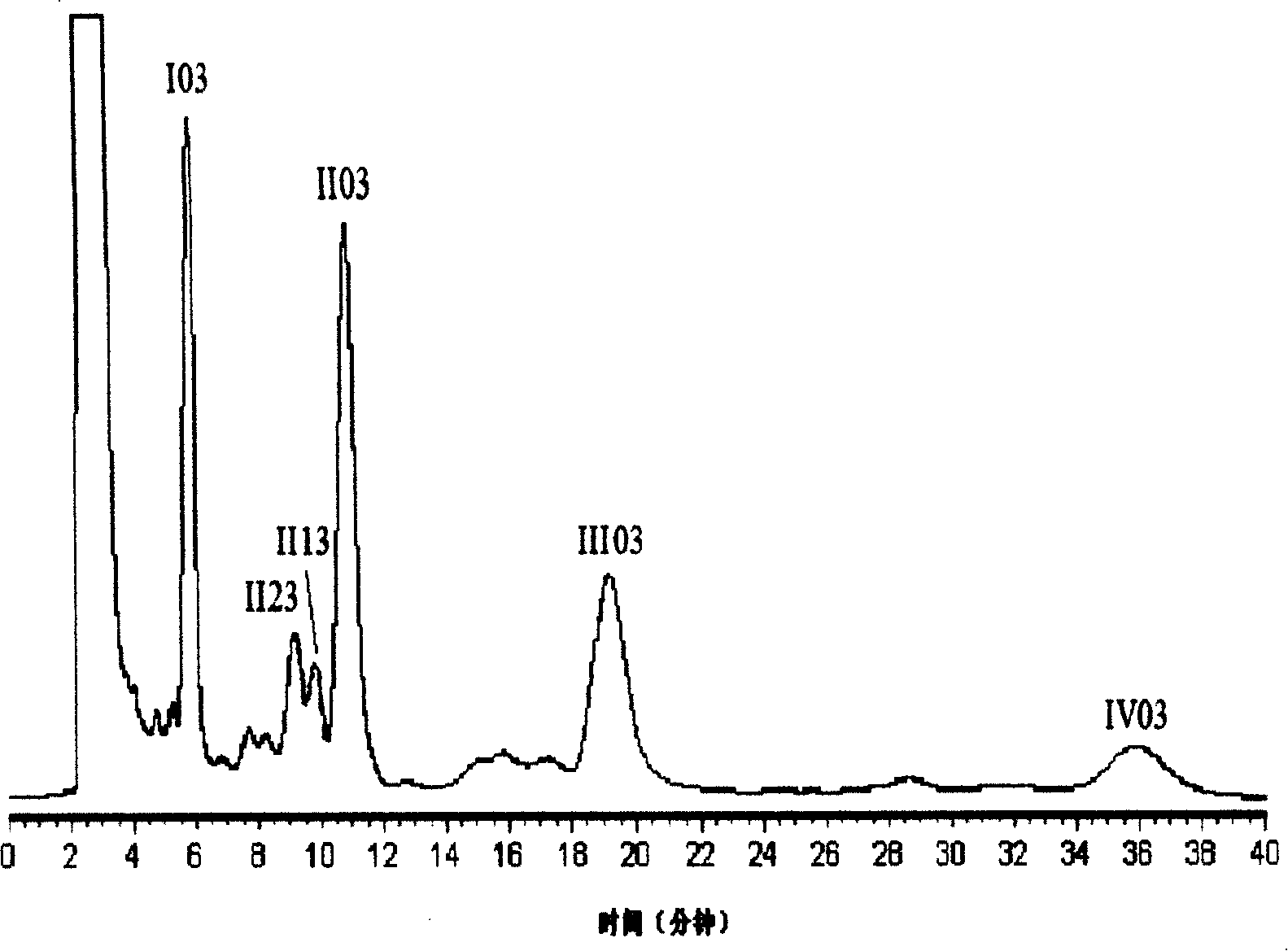
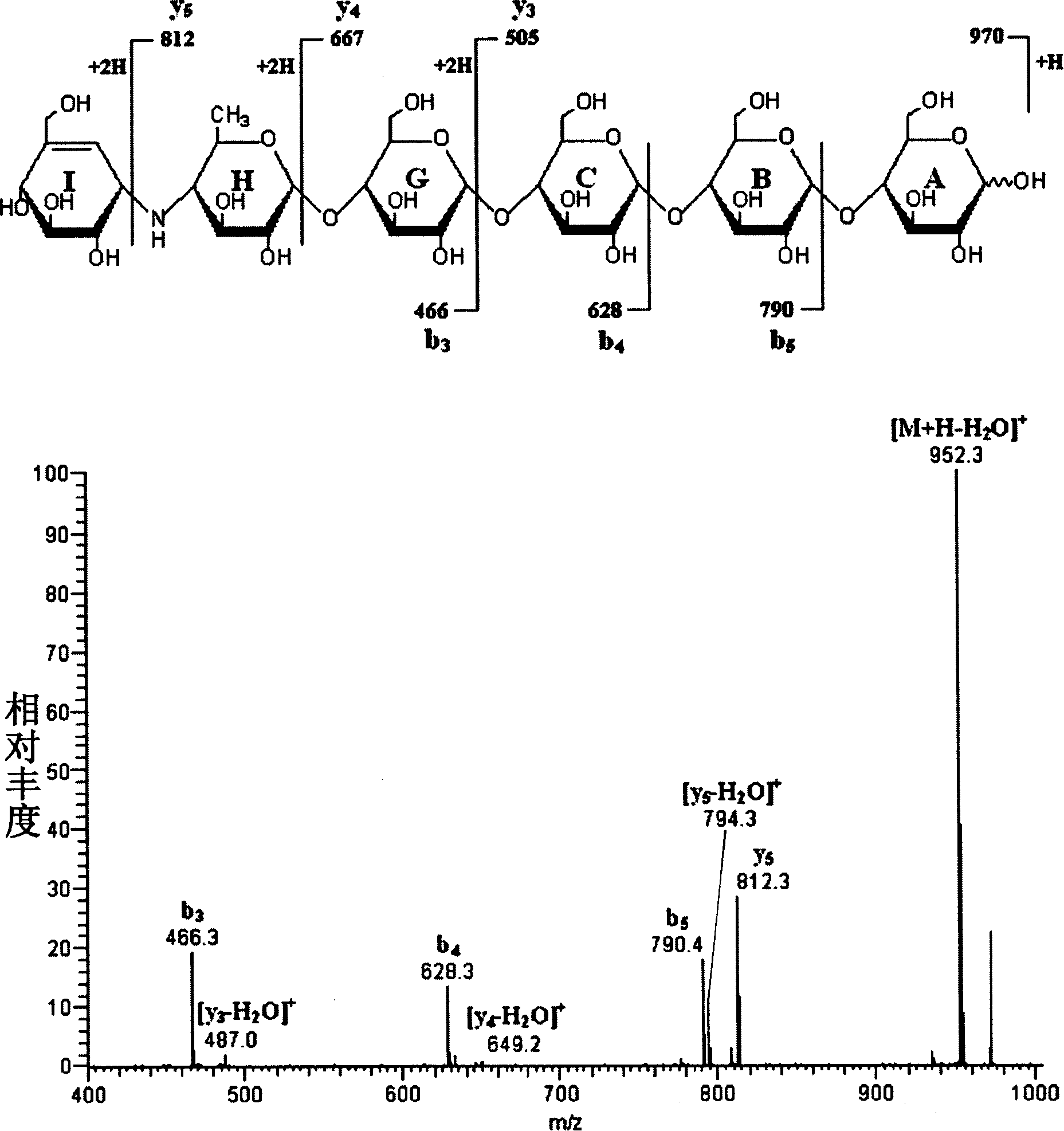
[0037] Example 2: Chemical structures of alcaftadine monomers I03, II03, III03, IV03.
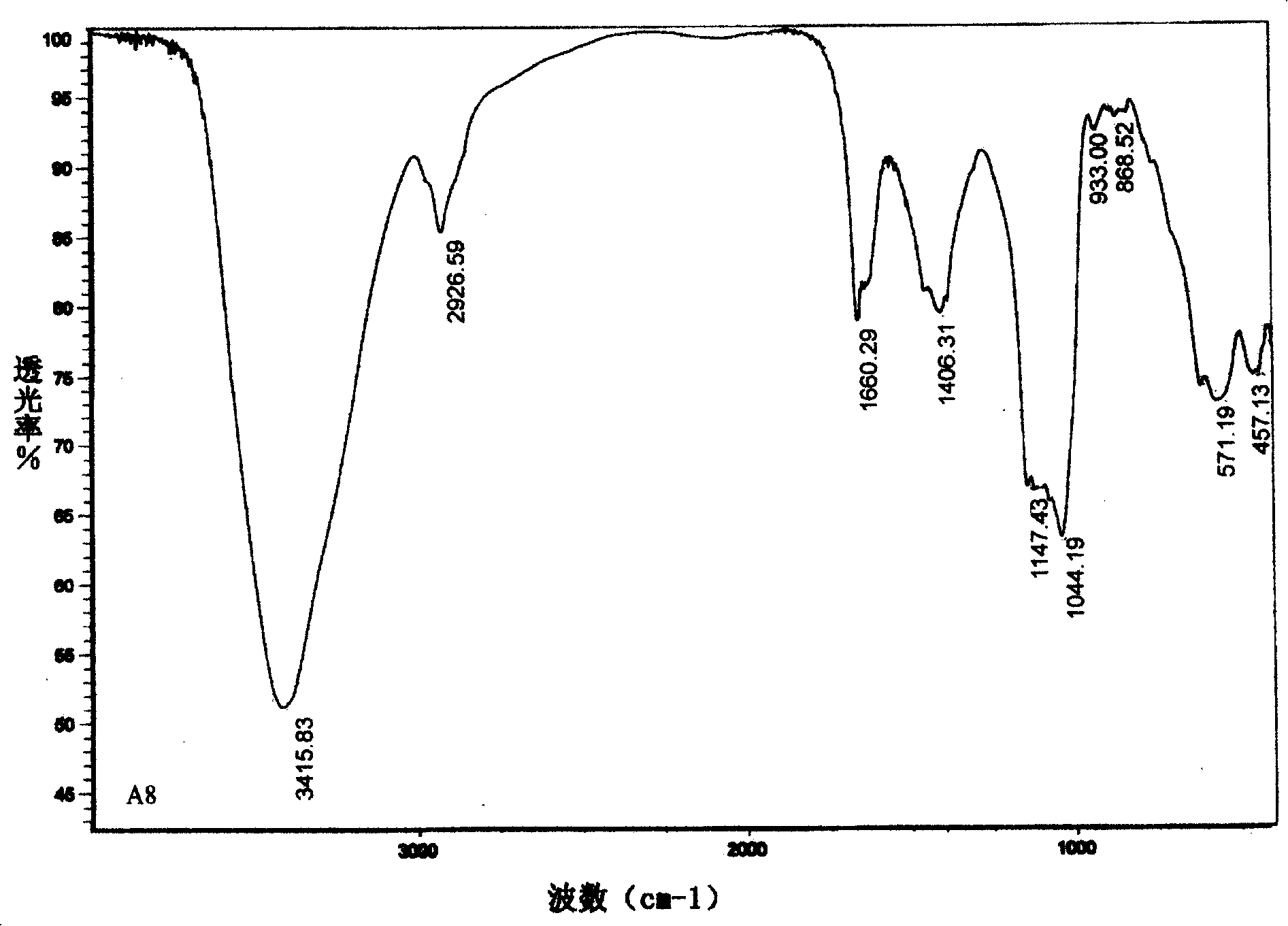
[0038] The physical and chemical properties of alcaftadine monomers I03, II03, III03, and IV03 are shown in Table 1. The molecular formula and molecular weight were analyzed by high-resolution mass spectrometry (+)ESI-FTMS. For the infrared spectrum of representative compound III03, see figure 2 .
[0039] Table 1. Physicochemical properties and molecular characteristics of alcaftadine
[0040] Alcatadine I03 Alcatadine II03 Alcatadine III03 Alcatadine IV03
[0041] Appearance White powder White powder White powder White powder
[0042] UV Spectrum End Absorption End Absorption End Absorption End Absorption
[0043] Infrared Spectrum 3385, 1029cm -1 3385, 1041cm -1 3416, 1044cm -1 3405, 1039cm -1
[0044] [α] 18 D +159° +165° +168° +153°
[0045] Molecular formula C 37 h 63 NO 28 C 56 h 94 N 2 o 40 C 75 h 125 N 3 o ...
Embodiment 3
[0062] Example 3: Alcaftadine inhibits α-amylase activity at the enzymatic level
[0063] With 0.05-2% soluble starch as the substrate, 450 μL of solutions containing different concentrations of substrates and different concentrations of α-amylase inhibitors were pre-incubated at 37°C for 10 minutes, and then 50 μL of 5 U / mL porcine pancreatic α-amylase ( Sigma company product) to start the reaction, sample 50 μL every 5 minutes, add 50 μL 3M NaOH to stop the reaction. The determination of reducing sugar is based on maltose, adding 75 μL of 1% 3,5-dinitrosalicylic acid to the sample, then bathing in boiling water for 5 minutes, and measuring the light absorption value at 490 nm after appropriate dilution. Taking time as the abscissa and reducing sugar production as the ordinate, the slope of the obtained straight line is the initial reaction velocity.
[0064] The double reciprocal curve and the Dixon curve plot showed that the inhibitory effects of the four monomers on porci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com