Antineoplastic sulfur-containing alkannin and naphthoquinones derivatives
A derivative, shikonin technology, applied in the field of derivatives in the field of medical technology, can solve the problems of limited application, toxicity, side effects, etc., and achieve the effect of inhibiting the growth of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
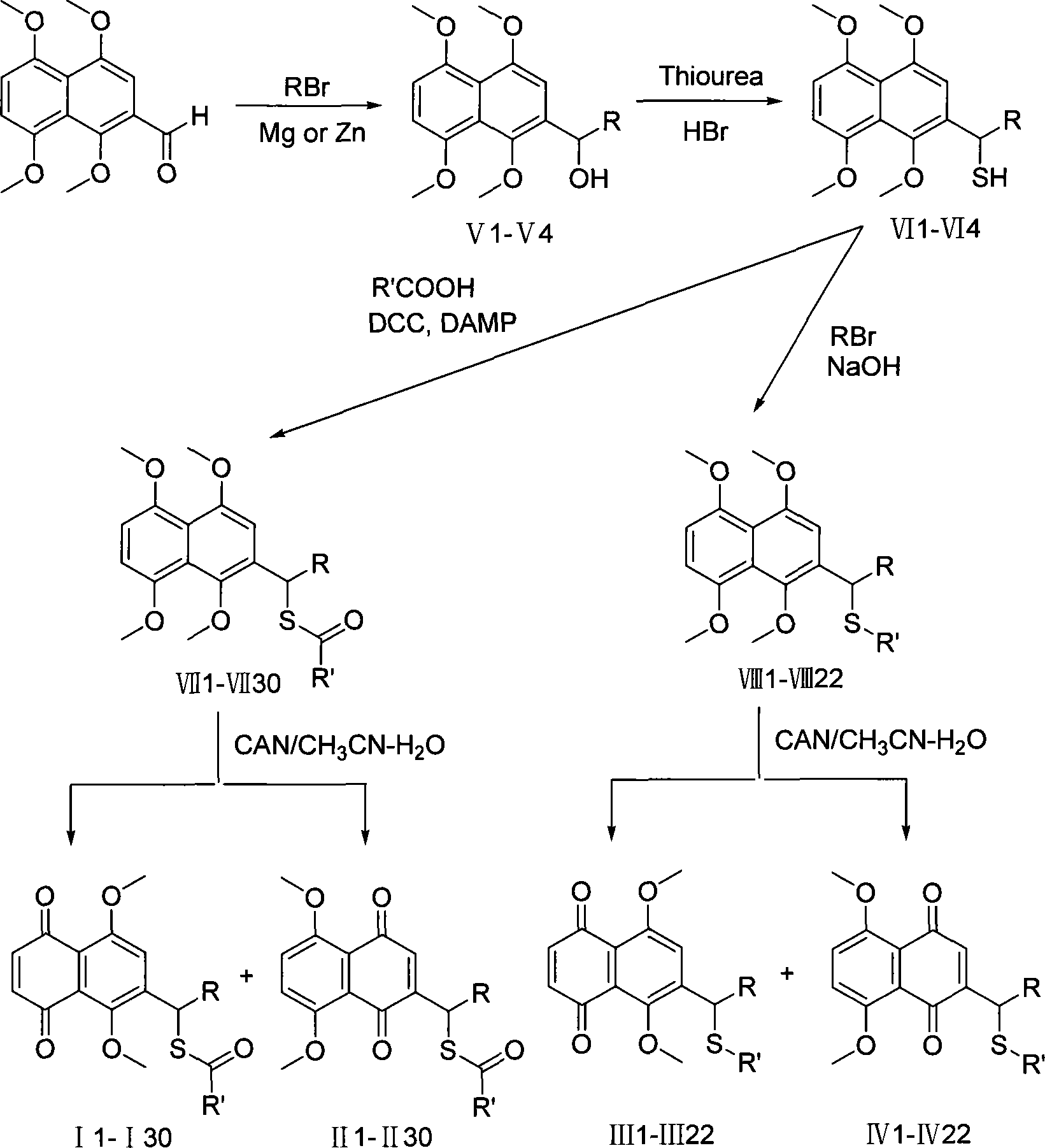
[0036] 2-(1-Hydroxy-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxynaphthalene V1
[0037] Under nitrogen protection, activated zinc powder (5 g, 0.077 mol) was placed in dry 100 ml tetrahydrofuran, bromoisoamyl (4 ml, 0.035 mol) was added dropwise, the reaction solution was stirred at room temperature for 1 hour, and the zinc powder was filtered off for later use.
[0038] 1,4,5,8-Tetramethoxynaphthalene-2-carbaldehyde (1.38g, 0.005mol) was dissolved in dry 50ml tetrahydrofuran, and the isopentenyl zinc bromide prepared above was added, and stirred at room temperature for 1 hour, Add 10ml of hexamethylphosphoric triamide, distill off tetrahydrofuran, and react at 130°C for 10 hours. After the reaction solution was cooled, a saturated aqueous ammonium chloride solution was added, extracted with ethyl acetate (30ml×3), washed with saturated brine, dried over anhydrous magnesium sulfate, and evaporated to obtain a crude product, which was separated by column chromatography (ether: n-h...
Embodiment 2
[0040] 2-(1-Mercapto-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxynaphthalene VI1
[0041]2-(1-hydroxy-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxynaphthalene V1 (0.87g, 0.025mol), thiourea (0.38g, 0.005mol) and 48% hydrobromic acid (0.56ml) were dissolved in 30ml of ethanol, and after reflux for 2 hours, the ethanol was evaporated, and 20ml of ether and 20ml of water were added. Under the protection of nitrogen, 10 ml of 40% NaOH solution was added dropwise to the aqueous layer, and after the addition was completed, it was refluxed for 2 h. Cool to room temperature, acidify with 6M hydrochloric acid, extract with dichloromethane (3×15ml), combine the organic layers, wash with saturated brine, dry over anhydrous magnesium sulfate, distill off the solvent to obtain a crude product, separate by column chromatography (ether: n-hexane alkane=1:5) to obtain 0.79 g of 2-(1-mercapto-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxynaphthalene (VI1), with a yield of 87%. 1 H NMR (CDCl 3 )δ: 7.01(...
Embodiment 3
[0043] Synthesis of 2-(1-alkanoylthio-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxynaphthalene VII1-VII15
[0044] 0.50mmol of 2-(1-mercapto-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxynaphthalene VI1 and 0.60mmol of carboxylic acid were dissolved in 10ml of dichloromethane, added DCC 0.75mmol and DMAP 0.25mmol were stirred overnight at room temperature under nitrogen. After the white precipitate was filtered off, the solvent was distilled off, and the crude product was separated by column chromatography to obtain a pale yellow oil.
[0045] 3.1.2-(1-acetylthio-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxynaphthalene VII1
[0046] Yield 94%. 1 H NMR (CDCl 3 )δ: 6.81(m, 3H), 5.29(t, J=7.2Hz, 1H), 5.05(t, J=7.2Hz, 1H), 3.92(s, 6H), 3.88(s, 3H), 3.78( s, 3H), 2.69 (m, 2H), 2.31 (s, 3H), 1.59 (s, 6H).
[0047] 3.2.2-(1-propionylthio-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxynaphthalene VII2
[0048] Yield 92%. 1 H NMR (CDCl 3 )δ: 6.80(m, 3H), 5.30(t, J=7.2Hz, 1H), 5.05(t, J=7.2Hz, 1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com