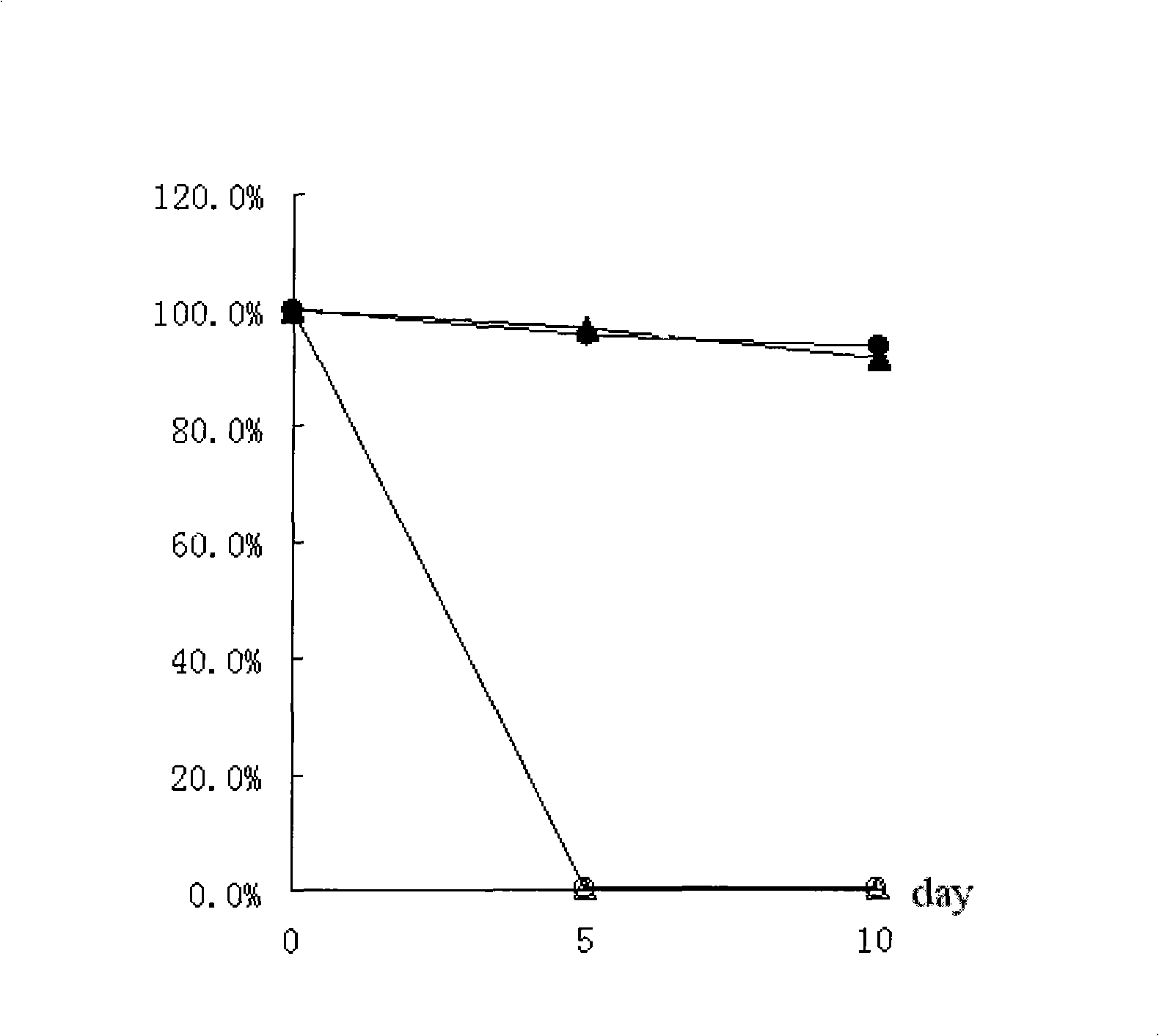Composite preparations containing double phosphinic acid compounds and vitamin D clathrate and method of preparing the same
A bisphosphonic acid compound and compound preparation technology, which is applied in the field of medicine, can solve problems such as difficult large-scale production, cumbersome process, and vitamin D degradation, and achieve improved solubility, improved stability and dissolution rate, and high safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
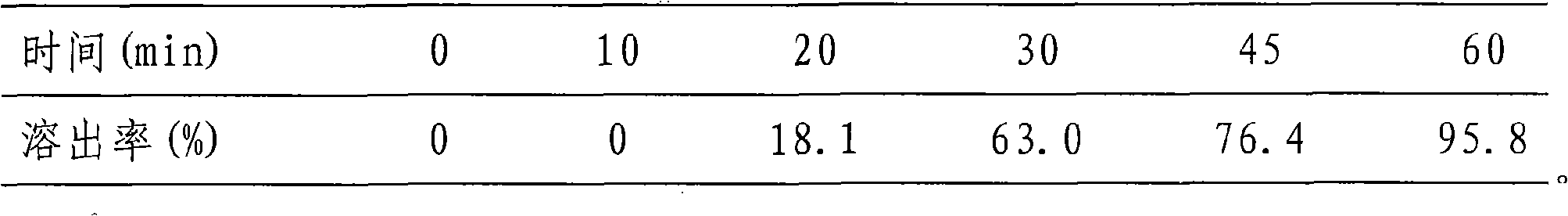
Examples
Embodiment 1
[0024] Vitamin D 3 Study on Stability of Inclusion Complex of -Hydroxypropyl-β-cyclodextrin
[0025] Take an appropriate amount of inclusion compound and non-inclusion vitamin D powder and spread it into a thin layer of 2 mm thick, seal it in a glass ampoule, keep away from light, place it at 60°C, and place it under an illumination of (4500±500) 1x. Take samples at 5 and 10d, observe the appearance and detect the content. The results are attached figure 1 As shown, the clathrate significantly improved the stability of vitamin D to light and heat.
Embodiment 2
[0027] Alendronate Sodium Vitamin D3 Compound enteric-coated tablet
[0028] Vitamin D 3 Preparation of -Hydroxypropyl-β-cyclodextrin inclusion compound
[0029] Vitamin D 3 Feed with refined hydroxypropyl-β-cyclodextrin at a mass ratio of 1:1000. Weigh the HP-β-CD and vitamin D of the prescription ratio 3 ; Dissolve vitamin D3 with a small amount of 95% ethanol, and place it in a suitable container away from light; configure HP-β-CD and deionized water to form a 10% HP-β-CD aqueous solution, and mix HP-β-CD under stirring conditions Slowly add the aqueous solution to the vitamin D 3 In the container of the solution, sonicate for 20 minutes. Filtered through a 0.45 μm microporous membrane, the resulting clear solution was pre-frozen in a -70°C refrigerator for 12 hours, and then freeze-dried in a vacuum freeze dryer for 15 hours. The dry matter is pulverized and passed through a 80-mesh sieve.
[0030] tablet preparation
[0031] prescription:
[0032] Component Dosag...
Embodiment
[0044] Alendronate Sodium Vitamin D 3 Compound Capsules
[0045] Vitamin D 3 Preparation of -Hydroxypropyl-β-cyclodextrin inclusion compound
[0046] Vitamin D 3 Feed with refined hydroxypropyl-β-cyclodextrin at a mass ratio of 1:1000. Weigh the HP-β-CD and vitamin D of the prescription ratio 3 ; Dissolve vitamin D with a small amount of 95% ethanol 3 , placed in a suitable container away from light; HP-β-CD and deionized water were configured to form a 10% HP-β-CD aqueous solution, and the HP-β-CD aqueous solution was slowly added to vitamin D under stirring conditions. 3 In the container of the solution, sonicate for 20 minutes. Filtered through a 0.45 μm microporous membrane, the resulting clear solution was pre-frozen in a -70°C refrigerator for 12 hours, and then freeze-dried in a vacuum freeze dryer for 15 hours. The dry matter is pulverized and passed through a 80-mesh sieve.
[0047] Capsule preparation
[0048] prescription:
[0049] Component Dosage
[005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com