Process for preparing and separating methyl docosapentaenoate and methyl docosahexenoate
A kind of technology of methyl docosahexaenoate and methyl docosahexaenoate, applied in the field of preparation and separation of methyl docosahexaenoate and methyl docosahexaenoate , which can solve the problems of complicated brine shrimp meal process and lower yield, and achieve the effect of short heating time, avoided loss and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Apply silver nitrate column method to prepare high-purity docosapentaenoic acid (DPA) methyl ester and docosahexaenoic acid (DHA) methyl ester, comprising the steps:
[0027] 1) Preparation of crude fatty acid: Take 500g of microalgae oil, 50g of KOH, and 1000ml of methanol, put them into a three-necked flask, use nitrogen as a protective gas, stir continuously, and reflux in a water bath at 60-120°C for 1-4 hours. After most of the solvent was removed by rotary evaporation, 6mol / L hydrochloric acid was added to acidify. After the fatty acid was extracted three times with 300ml petroleum ether, the ether phase was washed with 5% NaCl aqueous solution until the washing liquid was neutral. Add anhydrous Na 2 SO 4 Absorb the residual water in the ether phase, and remove the solvent by rotary evaporation at 60°C to obtain the crude fatty acid.
[0028] 2) Preparation of DPA and DHA mixed fatty acid: Take 100g crude fatty acid, 30g~300g urea, 600ml methanol and mix, reflux...
Embodiment 2
[0032] Basic technology is with embodiment 1, and concrete operation parameter is as follows:
[0033] 1) Preparation of crude fatty acid: Take 250 g of seaweed oil, 50 g of KOH, and 500 ml of methanol, put them into a three-necked flask, use nitrogen as a protective gas, keep stirring, and reflux in a water bath at 60-120° C. for 2 hours. After most of the solvent was removed by rotary evaporation, 6mol / L hydrochloric acid was added to acidify. After the fatty acid was extracted three times with 300ml petroleum ether, the ether phase was washed with 5% NaCl aqueous solution until the washing liquid was neutral. Add anhydrous Na 2 SO 4 Absorb the residual water in the ether phase, and remove the solvent by rotary evaporation at 60°C to obtain the crude fatty acid.
[0034] 2) Preparation of DPA and DHA mixed fatty acid: Take 75g of crude fatty acid, 150g of urea, and 600ml of methanol, mix them, reflux in a water bath at 50-80°C for 15 minutes, cool and crystallize at -10°C ...
Embodiment 3
[0038] Basic technology is with embodiment 1, and concrete operation parameter is as follows:
[0039] 1) The preparation of crude fatty acid, the methyl esterification of the preparation of DPA and DHA mixed fatty acid and DPA and DHA mixture are the same as embodiment 2.
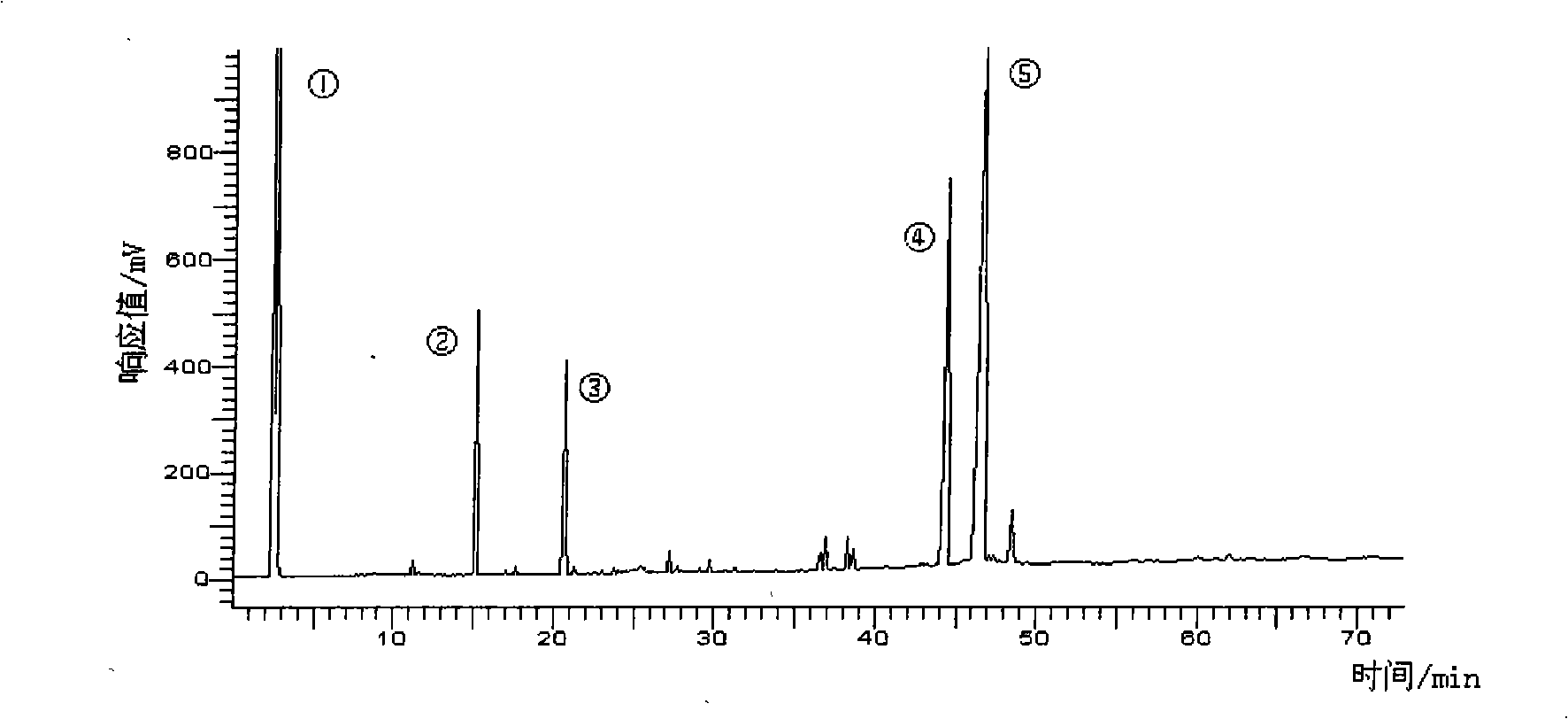
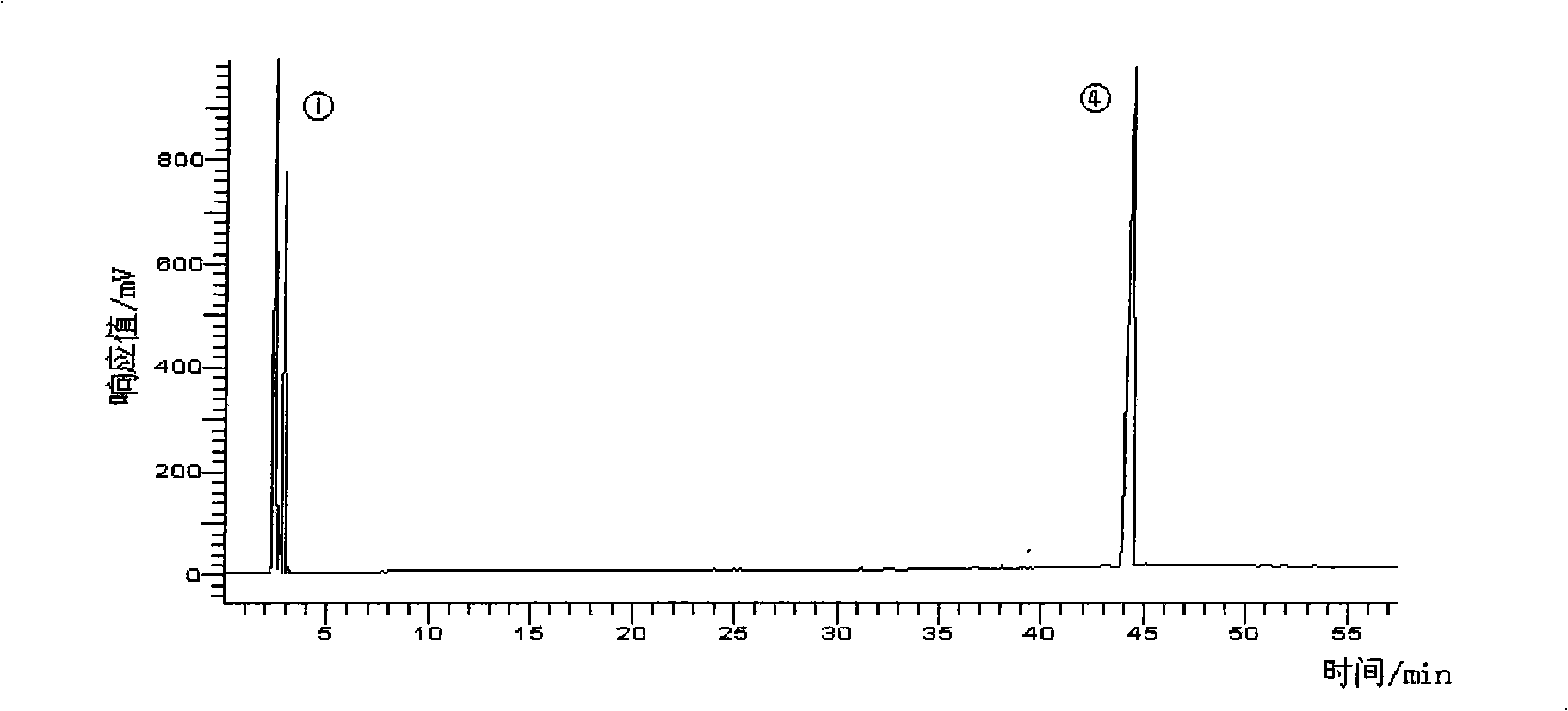
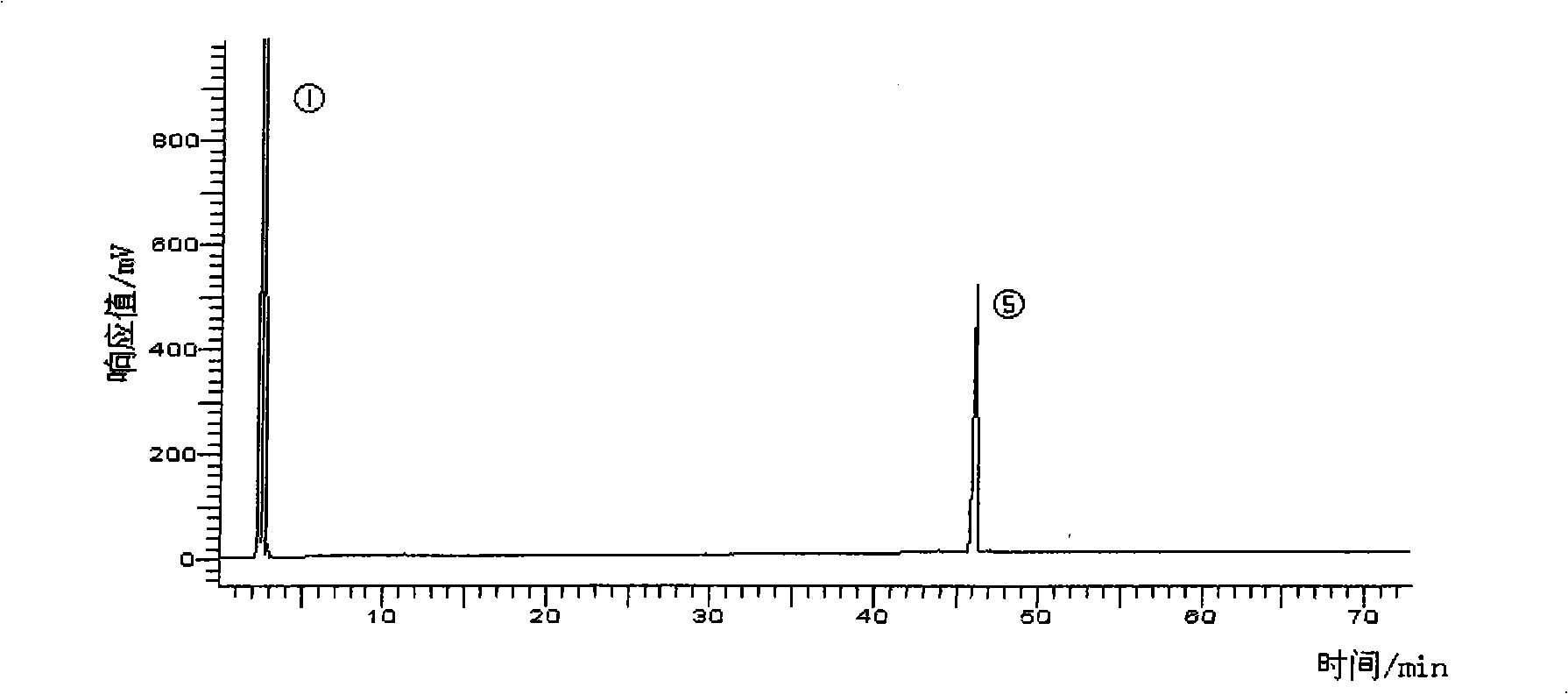
[0040] 2) silver nitrate column method obtains DPA methyl ester and DHA methyl ester respectively: prepare Ag by the method in example 1 + Silica gel, weigh 100g of the prepared Ag + Silica gel, wet packed column with n-hexane as eluent. Take DPA methyl ester and DHA methyl ester mixture 0.5g sample. Respectively with 0.5% acetone n-hexane solution 2000ml, 10% acetone n-hexane solution 500ml, 15% acetone n-hexane solution 1000ml elution, each 60ml as a sample, evaporated to remove solvent, detected by gas chromatography, combined the same group points, high-purity DPA methyl ester and DHA methyl ester can be obtained respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com