Substituted flavonoids and preparation method, application and pharmaceutical composition thereof
A technology of flavonoids, substituents, applied in the fields of medicinal chemistry and pharmacotherapeutics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
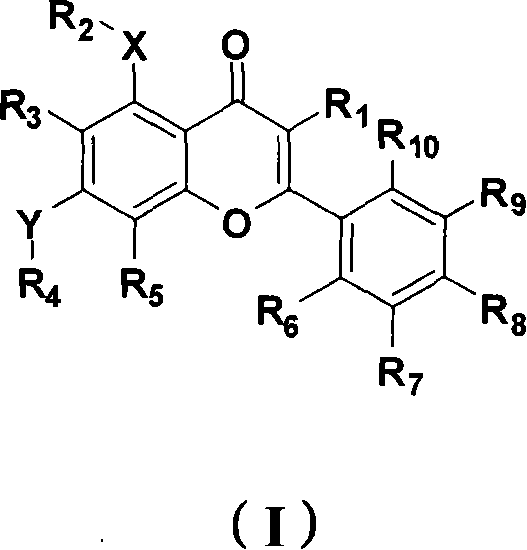
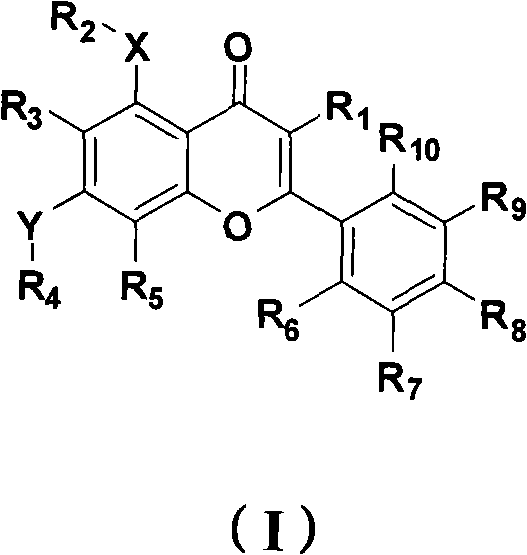
[0110] Synthesis of 5,7-dihydroxy-3-(3-methylbut-2-enyl)-2-phenyl-4H-chromen-4-one (compound 1):
[0111] Step (1): Dissolve 0.5g of ethyl 3-oxo-3-phenylpropionate in 5mL of DMF to obtain a colorless clear liquid, add 0.4g of potassium carbonate and 3,3-dimethylallyl bromide under stirring at room temperature 0.305mL, react overnight at room temperature. The next day, add 20 mL of water to the system, transfer to a separatory funnel, extract three times with 30 mL of ethyl acetate, combine the organic phases, wash twice with 25 mL of water, twice with 20 mL of saturated saline, and dry over anhydrous magnesium sulfate. The desiccant was filtered off, and the solvent was evaporated to dryness to obtain the crude product as viscous oil, which was purified by column chromatography to obtain 575 mg of the pure product, with a yield of 85%. 1 H NMR (CDCl 3 , 300M): δ8.24(d, J=7.2Hz, 2H), 7.57(t, J=7.2Hz, 1H), 7.45(t, J=7.5Hz, 2H), 5.10(t, J=7.2Hz , 1H), 4.29(t, J=7.5Hz, 1H), 4.1...
Embodiment 2
[0114] 5,7-dihydroxy-3-(3-methylbut-2-enyl)-2-(2,4,5-trimethoxyphenyl)-4H-chromen-4-one (compound 2) Synthesis of:
[0115] Step (1): According to the literature method [Kumazawa, T. et al. Carbohydr. Res. 2000, 329, 507.], the double MOM-protected trihydroxyacetophenone was prepared.
[0116]Step (2): Dissolve 3.1g of double-MOM-protected trihydroxyacetophenone in 20mL of anhydrous DMF, slowly drop 1.5g of sodium hydride in DMF solution under ice cooling, and then add 2,4,5-trimethoxy 3.0 g of benzoyl chloride was reacted overnight at room temperature. Add 50mL of cold water at 0-10°C to the system, extract with 50mL of ethyl acetate, combine the organic phases, wash with 50mL of water and 50mL of saturated brine, and dry over anhydrous magnesium sulfate. The desiccant was filtered off, and the product obtained by evaporating the solvent to dryness could be directly used in the next reaction with a yield of 97%. 1 H NMR (CDCl 3 , 300M): δ13.42(s, 1H), 7.56(s, 1H), 6.48(s,...
Embodiment 3
[0120] 5-Hydroxy-3-(3-methylbut-2-enyl)-4-oxo-2-(2,4,5-trimethoxyphenyl)-4H-benzopyran-7-substituted Synthesis of mesylate (compound 3):
[0121] Dissolve 100mg of compound 2 in 2ml of anhydrous tetrahydrofuran, add 20μL of methanesulfonyl chloride and 34μL of triethylamine under ice cooling, react at room temperature until the raw materials disappear, evaporate the solvent, add 10mL of water to the system, extract with 15mL of ethyl acetate, and the organic phase successively Wash twice with 20 mL of water and 20 mL of saturated saline, and dry over anhydrous magnesium sulfate. The desiccant was filtered off, and the solvent was evaporated to dryness to obtain a crude product, which was recrystallized from a mixed solvent of ethyl acetate and petroleum ether (V: 1 / 3) to obtain 107 mg of a pure product with a yield of 90%. 1 H NMR (CDCl 3 , 300M): δ13.15(s, 1H), 6.86(s, 1H), 6.84(s, 1H), 6.66(s, 1H), 6.60(s, 1H), 5.07(t, J=6.6Hz, 1H), 3.97(s, 3H), 3.85(s, 3H), 3.79(s, 3H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com