Catalyst for producing tetrafluoromethane with gas-phase fluoridation and production method
A tetrafluoromethane, gas phase fluorination technology, applied in the direction of halogen substitution preparation, etc., can solve the problems of poor activity and low stability, and achieve the effects of fast price, high catalytic activity or selectivity, and high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
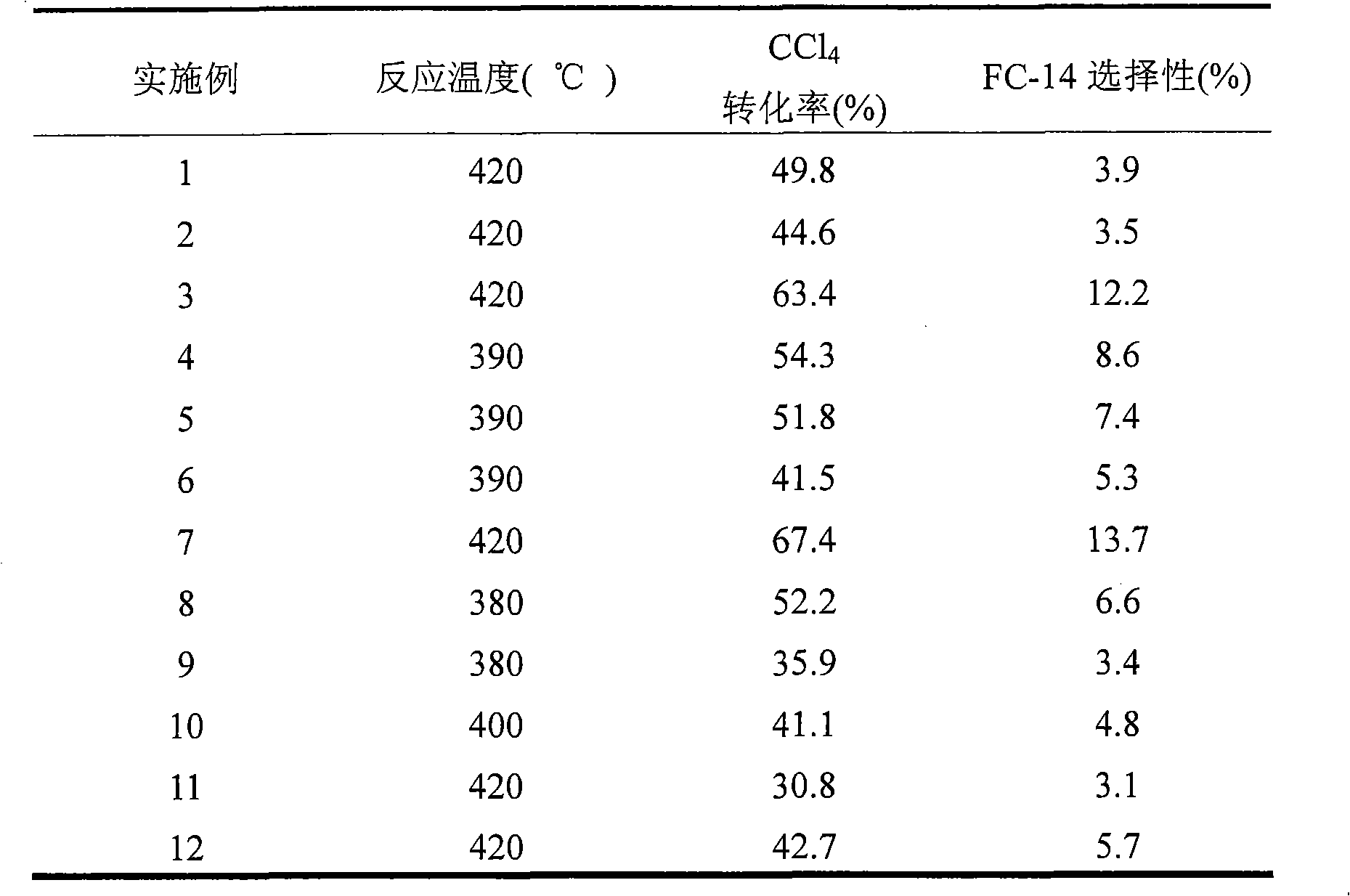
[0029] First, weigh CrCl according to the loading amount of Cr as 70wt%. 3 and Y(OH) 3 , mix well with an appropriate amount of water, add a certain amount of Na with a concentration of 2mol / L 2 CO 3 After the precipitation is complete, it is separated and washed until the filtrate is neutral, and then the precipitate is dried at 90°C. The dried precipitate was molded and then heated at 400°C under N 2Calcination was carried out in the atmosphere for 12 hours to obtain a fluorinated catalyst precursor. The fluorination catalyst precursor was treated with anhydrous HF at 600° C. for 2 hours to obtain a fluorination catalyst. Its reactivity or selectivity are shown in Table 1, Table 2, Table 3, Table 4.
Embodiment 2
[0031] First, weigh Cr according to the loading amount of Cr being 20wt%, and the doping amount of Fe being 2wt%. 2 o 3 , FeCl 3 and Y 2 o 3 , mix evenly with an appropriate amount of water, add a certain amount of ammonia water with a concentration of 2mol / L until the precipitation is complete, then separate and wash until the filtrate is neutral, and then dry the precipitate at 140°C. After drying, the precipitate was molded at 650°C and N 2 Calcination was carried out in the atmosphere for 2 hours to obtain a fluorinated catalyst precursor. The fluorination catalyst precursor was treated with anhydrous HF at 380° C. for 24 hours to obtain a fluorination catalyst. Its reactivity or selectivity are shown in Table 1, Table 2, Table 3, Table 4.
Embodiment 3
[0033] First, weigh Cr(OH) according to the loading amount of Cr being 50wt%, and the doping amount of Ga being 3wt%. 3 , Ga 2 o 3 and Y(NO 3 ) 3 , mix well with an appropriate amount of water, add a certain amount of 2mol / L (NH 4 ) 2 CO 3 After the precipitation is complete, it is separated and washed until the filtrate is neutral, and then the precipitate is dried at 120°C. After the dried precipitate was molded, it was heated at 250°C and H 2 Calcined in the atmosphere for 48 hours to obtain a fluorinated catalyst precursor. The fluorination catalyst precursor was treated with anhydrous HF at 500° C. for 8 hours to obtain a fluorination catalyst. Its reactivity or selectivity are shown in Table 1, Table 2, Table 3, Table 4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com