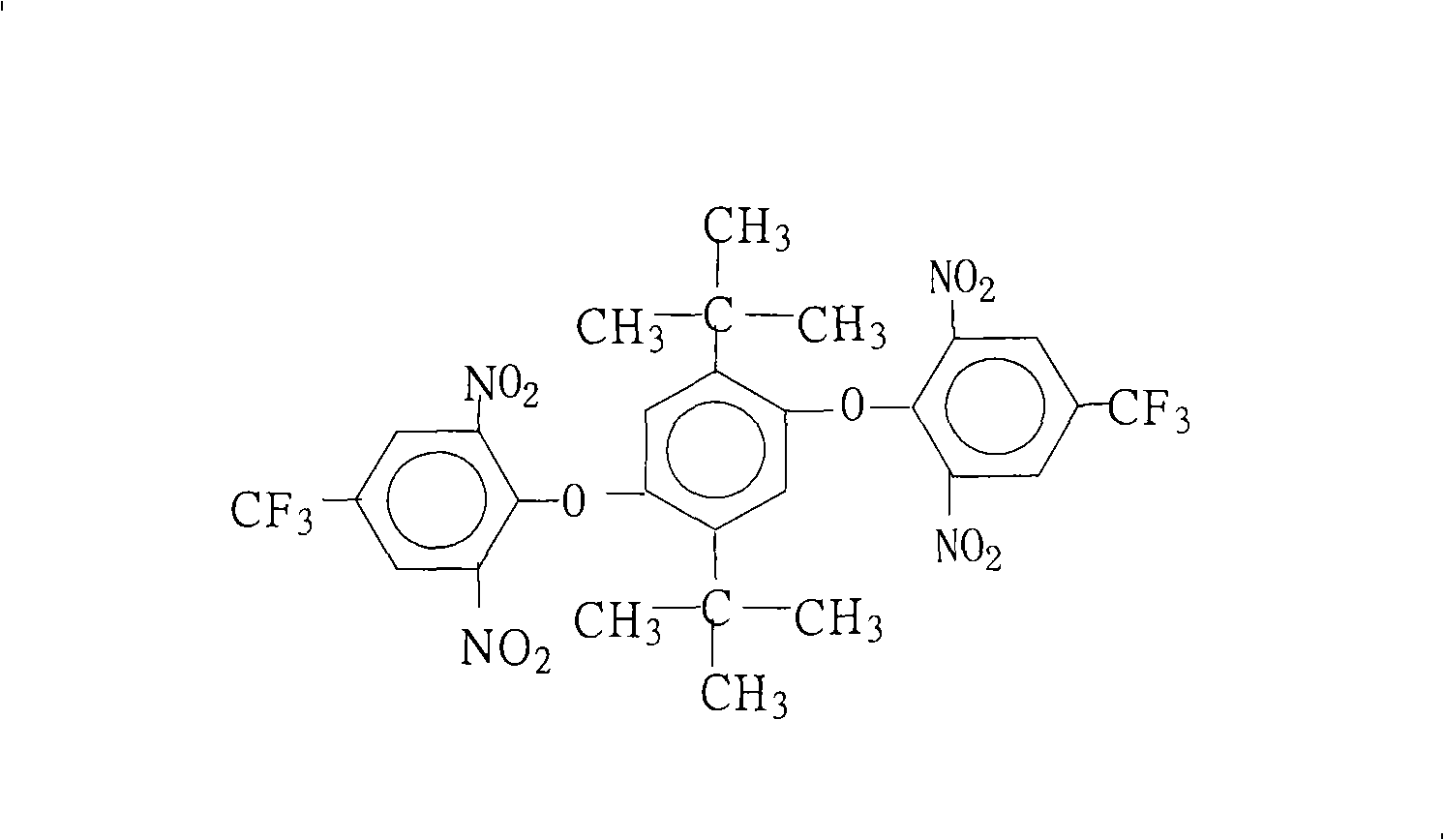
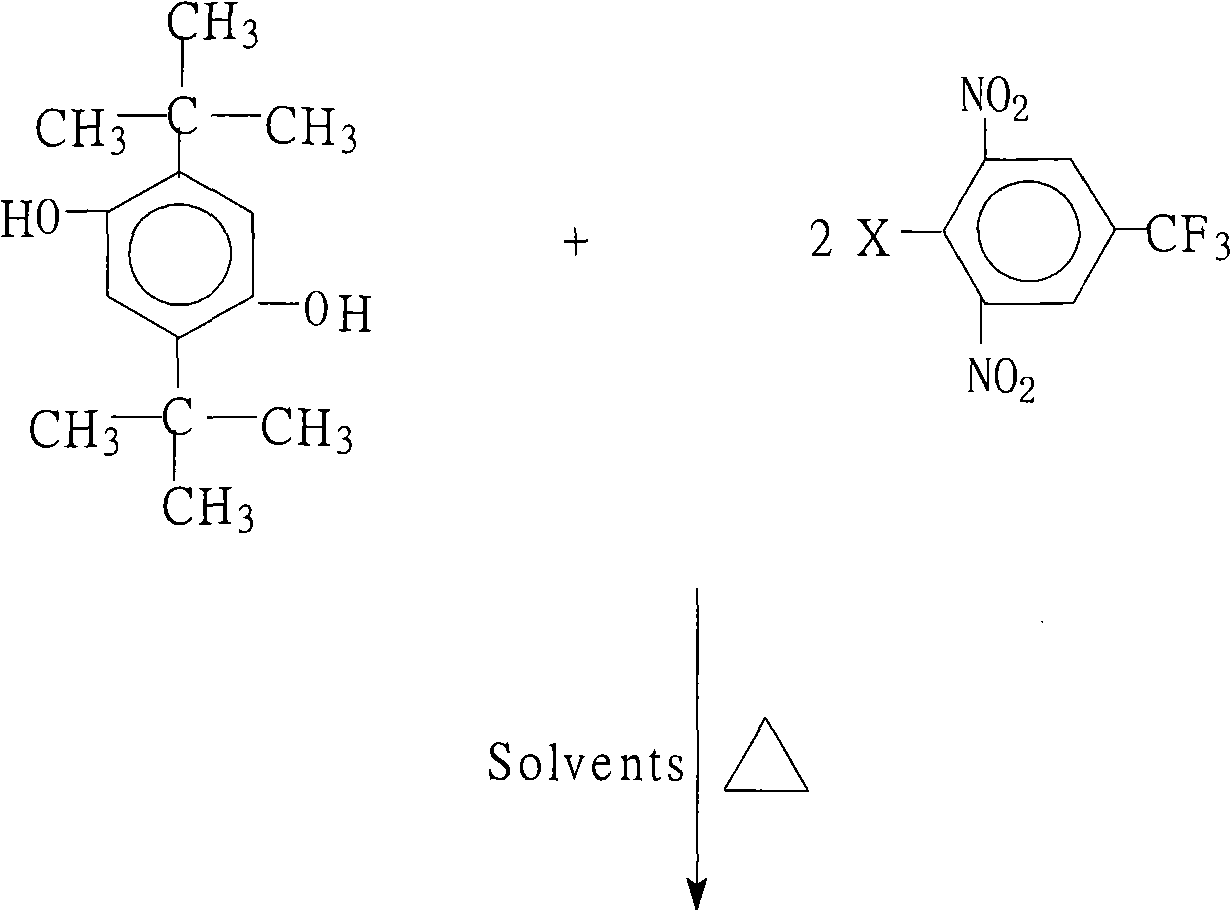
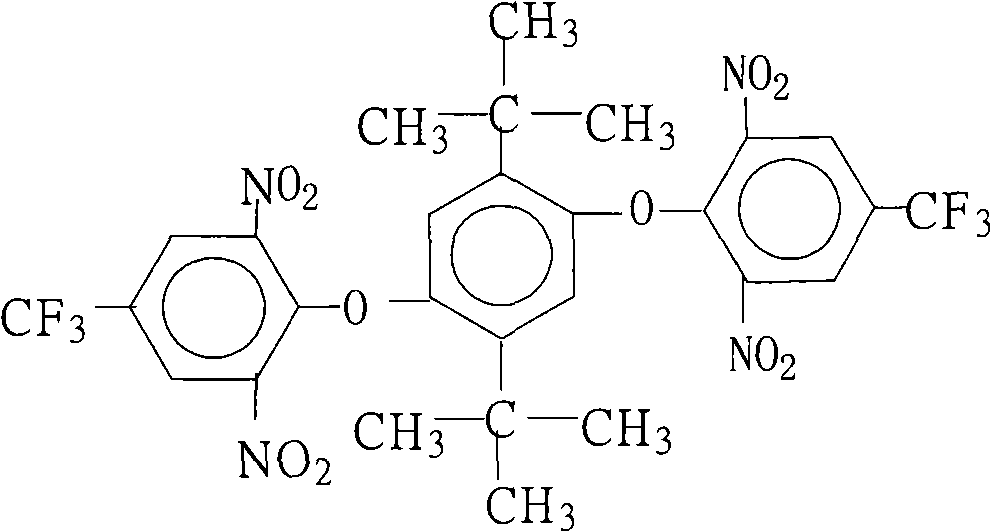
Method for preparing 1,4-bis(2,6-di-nitryl-4-trifluoromethyl phenoxy)-2,5 bi-utylbenzene
A technology of trifluoromethylphenoxy and trifluoromethylhalogenated benzene, which is applied in the field of preparation of aromatic organic compounds, can solve the problems of unpublished patents or literature reports, and achieve less types of use and less waste , the effect of convenient recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 22.2 grams (0.10 moles) of 2,5-di-tertbutylhydroquinone, 54.1 grams (0.20 moles) of 2,6-dinitro-4-trifluoromethylchlorobenzene, 110.4 grams (0.80 moles) Potassium carbonate, 1000 milliliters of N, N-dimethylformamide, 50 milliliters of benzene and 50 milliliters of toluene were put into the reaction kettle, stirred, heated to reflux and separated from water for 6 hours, concentrated the reaction solution, recovered the solvent for recycling, cooled The reactant system was added with water to precipitate a solid product, washed 2 to 3 times with hot water, and dried to obtain 64.3 grams of 1,4-bis(2,6-dinitro-4-trifluoromethylphenoxy)- 2,5-Di-tert-butylbenzene crystal product with a purity of 99.7%. According to the actual amount of 1,4-bis(2,6-dinitro-4-trifluoromethylphenoxy)-2,5-di-tert-butylbenzene and the theoretical amount (69.0 grams), the calculation of 1 , the yield of 4-bis(2,6-dinitro-4-trifluoromethylphenoxy)-2,5-di-tert-butylbenzene was 93.2%.
Embodiment 2
[0030] 22.2 grams (0.10 moles) of 2,5-di-tertbutyl hydroquinone, 69.3 grams (0.22 moles) of 2,6-dinitro-4-trifluoromethylbromobenzene, 10.0 grams (0.10 moles) Potassium bicarbonate, 55.2 grams (0.40 moles) of potassium carbonate, 650 milliliters of N-methyl-2-pyrrolidone, 50 milliliters of N, N-dimethylacetamide and 1000 milliliters of toluene were put into the reactor, stirred, heated to reflux After reacting with water for 12 hours, concentrate the reaction solution, recover the solvent for recycling, cool the reactant system, add water, and precipitate a solid product, wash with hot water 2 to 3 times, and dry to obtain 61.5 grams of 1,4-bis(2,6 - A crystalline product of dinitro-4-trifluoromethylphenoxy)-2,5-di-tert-butylbenzene with a purity of 99.8%. According to the actual amount of 1,4-bis(2,6-dinitro-4-trifluoromethylphenoxy)-2,5-di-tert-butylbenzene and the theoretical amount (69.0 grams), the calculation of 1 , the yield of 4-bis(2,6-dinitro-4-trifluoromethylphenox...
Embodiment 3
[0032] 22.2 grams (0.10 moles) of 2,5-di-tertbutylhydroquinone, 54.1 grams (0.20 moles) of 2,6-dinitro-4-trifluoromethylchlorobenzene, 10.6 grams (0.10 moles) Sodium carbonate, 40 ml of N-methyl-2-pyrrolidone, 700 ml of benzene and 100 ml of dichlorobenzene were put into the reaction kettle, stirred, heated to reflux and separated from water for 13 hours, the reaction solution was concentrated, and the solvent was recovered for recycling , cooling the reactant system, adding water, the solid product was separated out, washed 2 to 3 times with hot water, and dried to obtain 51.4 grams of 1,4-bis(2,6-dinitro-4-trifluoromethylphenoxy) -2,5-di-tert-butylbenzene crystal product with a purity of 99.2%, according to the actual obtained 1,4-bis(2,6-dinitro-4-trifluoromethylphenoxy)-2,5 - Amount and theoretical yield (69.0 g) of di-tert-butylbenzene, calculated to give 1,4-bis(2,6-dinitro-4-trifluoromethylphenoxy)-2,5-di-tert-butyl The yield of phenylbenzene was 74.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com