Phenyl s-triazine ligand for metallic organic frame complex and synthesizing process therefor
A technology of metal-organic frameworks and synthesis methods, applied in organic chemistry and other fields, can solve the problems of rare ligand research, long reaction time, and low yield, and achieve the effects of high yield, low cost, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
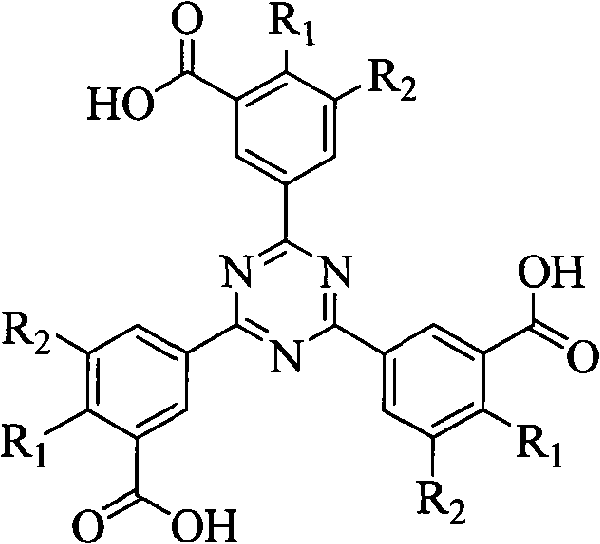
[0020] R in the structural formula of phenyl-s-triazine ligand 1 for hydrogen, R 2 For carboxyl.
[0021] The synthetic route of this phenyl-s-triazine ligand is as follows:
[0022]
[0023] The synthesis method is:
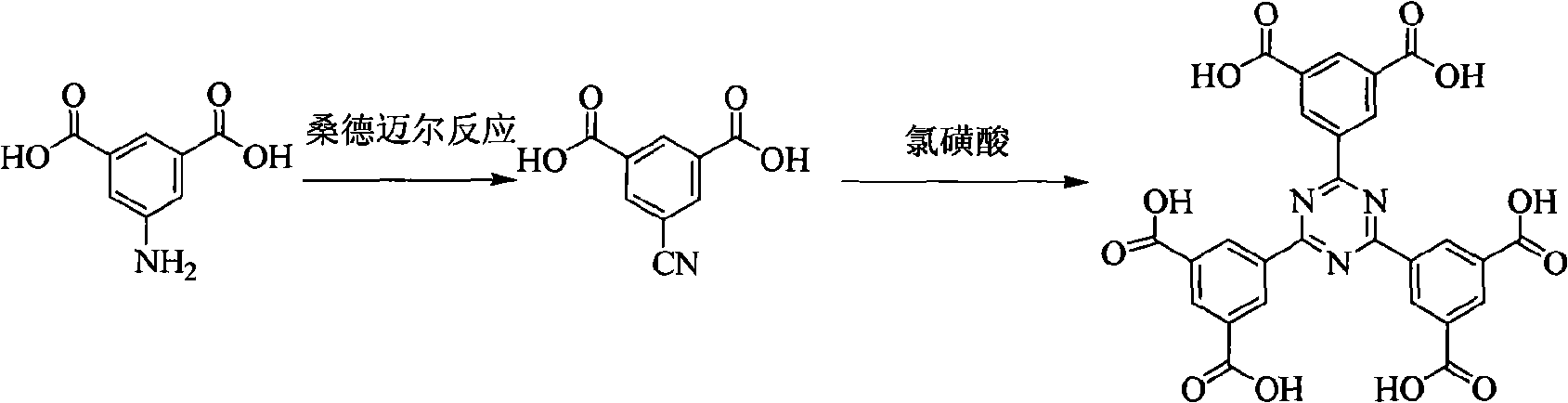
[0024] (1) Add 500ml of water and 2mol of concentrated hydrochloric acid to 1mol of 5-aminoisophthalic acid, and slowly add 200ml of NaNO with a molar concentration of 4mol / L at 0°C 2 aqueous solution for diazotization reaction. After reacting for 20 minutes, crushed ice and sodium carbonate were added to adjust the pH to 3-4 to obtain a diazonium salt solution.
[0025] (2) Take another beaker and add 1 mol of cuprous cyanide and 1 mol of sodium cyanide, and add a sufficient amount of water, after completely dissolving, add 2 mol of sodium carbonate, and cool to 0°C. Add the diazonium salt solution obtained in step (1) slowly, and react at 0° C. for 30 minutes under stirring, and then react at 50° C. for 5 hours.
[0026] (3) Add the reaction solution ...
Embodiment 2
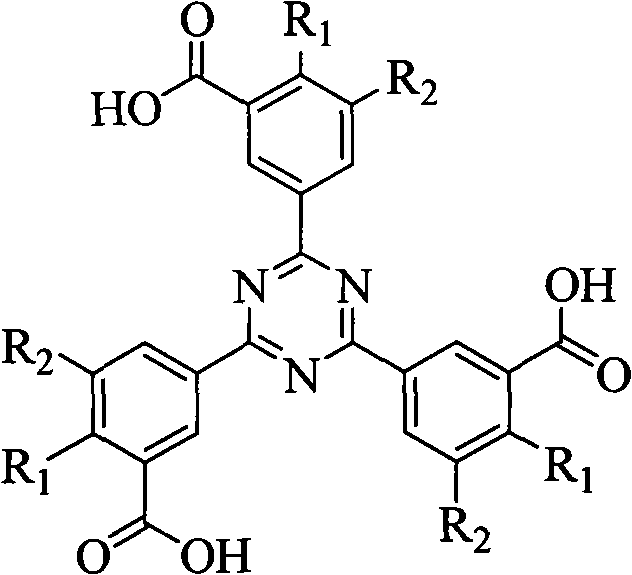
[0032] R in the structural formula of phenyl-s-triazine ligand 1 is the carboxyl group, R 2 for hydrogen.
[0033] The synthetic route of this phenyl-s-triazine ligand is as follows:
[0034]
[0035] The synthesis method is:
[0036] 1. Add 500ml of water and 4mol of concentrated hydrochloric acid to 1mol of 5-aminophthalic acid, and slowly add 200ml of NaNO with a molar concentration of 7mol / L at 5°C 2 aqueous solution for diazotization reaction. After reacting for 40 minutes, crushed ice and sodium carbonate were added to adjust the pH to 3-4 to obtain a diazonium salt solution.
[0037] 2. Take another beaker and add 1 mol of cuprous cyanide and 3 mol of sodium cyanide, and add sufficient amount of water. After completely dissolving, add 4 mol of sodium carbonate and cool to 0°C. Add the diazonium salt solution obtained in step (1) slowly, and react at 0° C. for 100 min under stirring, and then react at 90° C. for 2 h.
[0038] 3. Add the reaction liquid obtained ...
Embodiment 3
[0042] R in the structural formula of phenyl-s-triazine ligand 1 is methyl, R 2 for hydrogen.
[0043] The synthetic route of this phenyl-s-triazine ligand is as follows:
[0044]
[0045] The synthesis method is:
[0046] 1. Add 500ml of water and 3mol of concentrated hydrochloric acid to 1mol of 2-methyl-5-aminobenzoic acid, and slowly add 200ml of NaNO with a molar concentration of 5mol / L at 2°C 2 aqueous solution for diazotization reaction. After reacting for 30 minutes, crushed ice and sodium carbonate were added to adjust the pH to 3-4 to obtain a diazonium salt solution.
[0047] 2. Take another beaker and add 1 mol of cuprous cyanide and 2 mol of sodium cyanide, and add a sufficient amount of water. After completely dissolving, add 3 mol of sodium carbonate and cool to 0°C. Add the diazonium salt solution obtained in step (1) slowly, and react at 0° C. for 60 minutes under stirring, and then react at 70° C. for 3 hours.
[0048] 3. Add the reaction liquid obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com