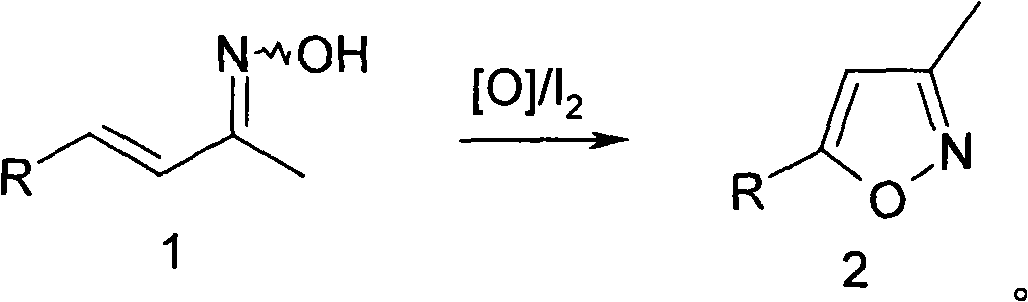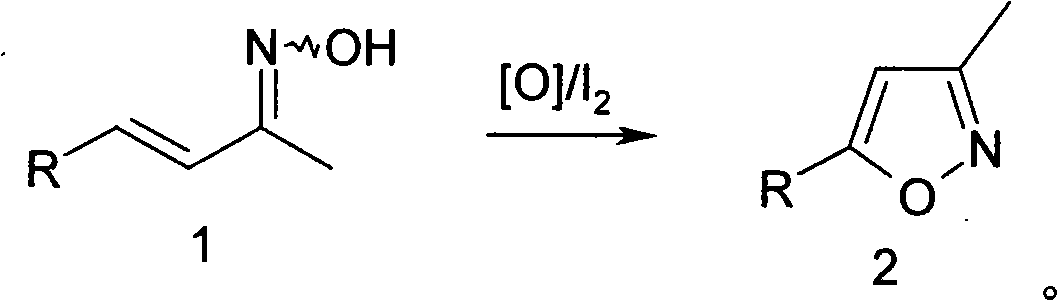Method for synthesizing isoxazole
A technology for isoxazoles and compounds, applied in the field of synthesizing isoxazoles, can solve the problems of unsuitability for industrial production, high cost, low yield and the like, and achieves the effects of eliminating the intermediate separation process, low equipment requirements, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 3-liter reaction flask, add 50 grams of α-ionone oxime and 500 milliliters of tetrahydrofuran, then add 100 milliliters of water and 8.5 grams of sodium bicarbonate, then add 0.5 grams of iodine, stir rapidly and heat to 50 ° C, maintain the temperature, slowly Slowly add 120 ml of 5% hydrogen peroxide dropwise, continue to react for 10 hours after the addition, TLC detects that the reaction is complete, add 5 grams of sodium bisulfite, stir for half an hour, the potassium iodide-starch test paper does not develop color, cool to room temperature, add 500 ml Ethyl acetate, stand still for half an hour, separate the organic layer, dry over anhydrous sodium sulfate, distill off the solvent, fractionate under reduced pressure, collect 100~110°C / 2mmHg fractions to obtain 35 grams of product, yield 76%.
Embodiment 2
[0021] The operation was the same as in Example 1, except that α-ionone oxime was replaced by β-ionone oxime, and 79% of the product was obtained.
Embodiment 3
[0023] In a 3-liter reaction flask, add 50 grams of α-ionone oxime and 500 milliliters of tetrahydrofuran, then add 160 milliliters of water and 8.5 grams of sodium bicarbonate, then add 5 grams of iodine, stir rapidly and heat to 50 ° C, maintain the temperature, slowly Slowly add 200 milliliters of 10% chlorine dioxide aqueous solution dropwise, continue to react for 10 hours after adding, TLC detects that the reaction is complete, add 5 grams of sodium bisulfite, stir for half an hour, potassium iodide-starch test paper does not develop color, cool to room temperature, Add 500 ml of ethyl acetate, stand still for half an hour, separate the organic layer, dry over anhydrous sodium sulfate, evaporate the solvent, fractionate under reduced pressure, collect 100-110°C / 2mmHg fractions, and obtain 40 g of the product with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com