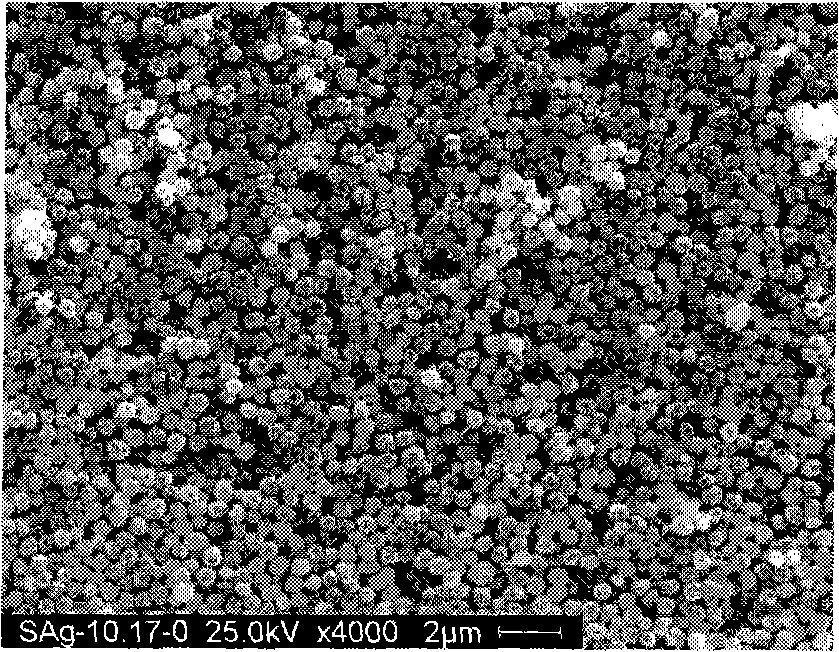
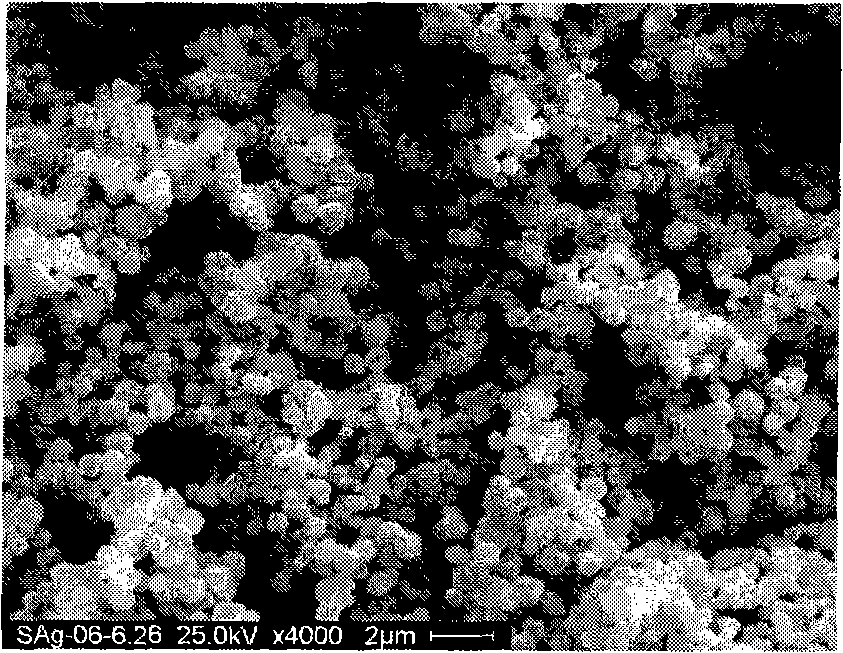
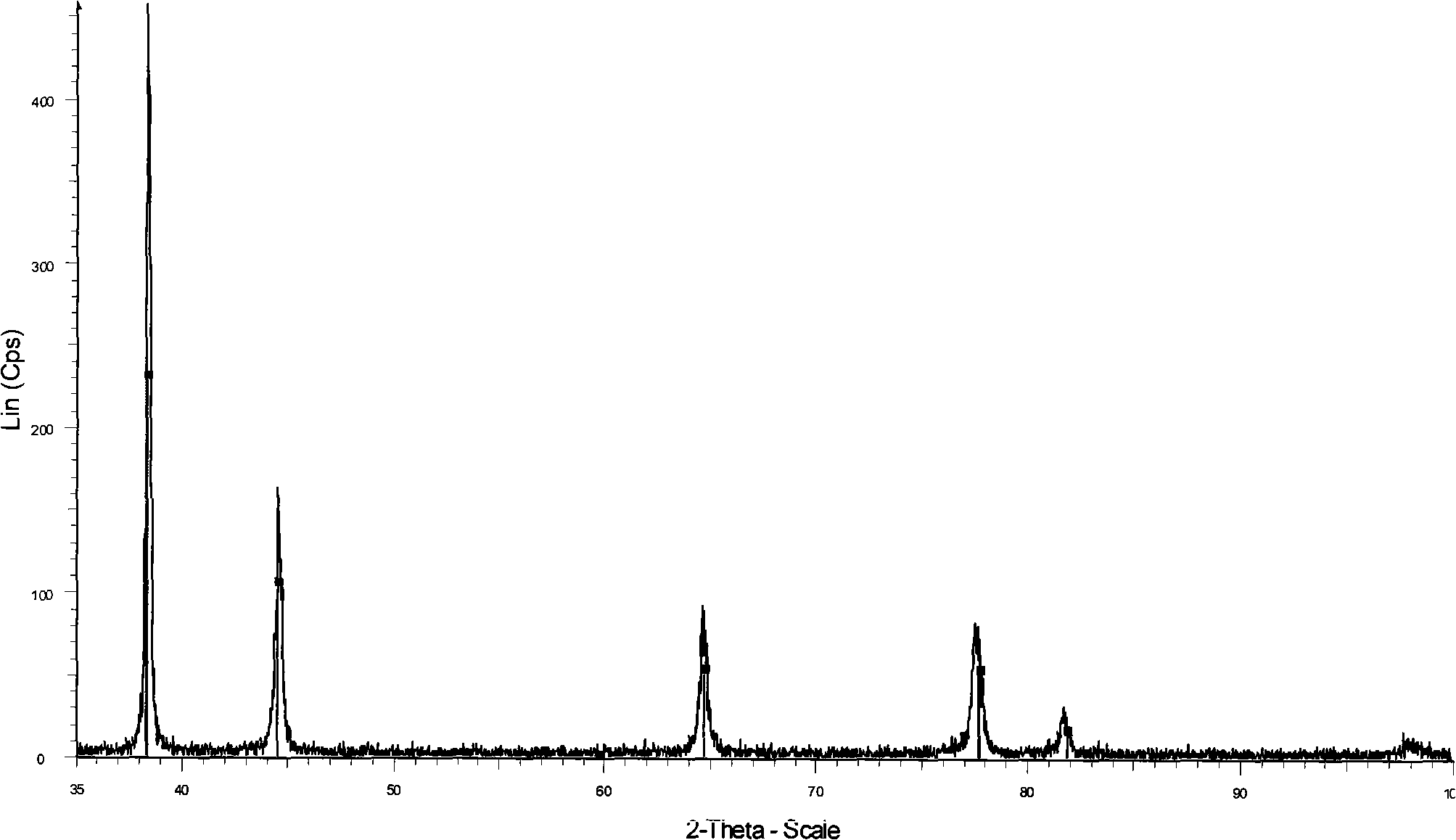
Preparation of high dispersed superfine spherical silver powder for conductive silver slurry
A conductive silver paste, high-dispersion technology, applied in the field of preparation of highly dispersed ultra-fine spherical silver powder for conductive silver paste, can solve the problem of inability to obtain high-dispersion ultra-fine silver powder, difficulties in silver powder collection and secondary dispersion, and inability to achieve ultra-fine Problems such as powder interception, to achieve the effect of stable and reliable process conditions, high yield and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1) Weigh 200g of analytically pure AgNO 3 Dissolve it in 3.6L of deionized water to prepare a silver nitrate solution; add 150-200ml of 25-28% ammonia water dropwise into the silver nitrate solution until the solution is just clear to obtain a silver-ammonia solution.
[0029] 2) Weigh 1.8g analytically pure NaOH and dissolve it in 12L deionized water to make solution A; weigh 18g PVA and dissolve it in 3L deionized water to make solution B; pour the prepared solutions A and B into the reaction kettle respectively , and measure 180mL of analytically pure formaldehyde into the reaction kettle, and stir for several minutes until the entire reduction system is a homogeneous phase.
[0030] 3) Add the silver ammonia solution dropwise to the reducing system, and control the adding rate to 100mL / min. After the silver ammonia solution is added dropwise, it is aged for 45 minutes to obtain a highly dispersed ultrafine silver powder slurry. The temperature of the whole reactio...
Embodiment 2
[0034] 1) Weigh 200g of analytically pure AgNO 3 Dissolve it in 3.6L of deionized water to prepare a silver nitrate solution; add 150-200ml of 25-28% ammonia water dropwise into the silver nitrate solution until the solution is just clear to obtain a silver-ammonia solution.
[0035] 2) Weigh 1.8g analytically pure NaOH and dissolve it in 12L deionized water to make solution A; weigh 18g PVA and dissolve it in 3L deionized water to make solution B; pour the prepared solutions A and B into the reaction kettle respectively , and measure 180mL of analytically pure formaldehyde into the reaction kettle, and stir for several minutes until the entire reduction system is a homogeneous phase.
[0036] 3) Add the silver ammonia solution dropwise to the reducing system, and control the adding rate to 100mL / min. After the silver ammonia solution is added dropwise, it is aged for 45 minutes to obtain a highly dispersed ultrafine silver powder slurry. The temperature of the whole reactio...
Embodiment 3
[0039] 1) Weigh 200g of analytically pure AgNO 3 Dissolve it in 3.6L of deionized water to prepare a silver nitrate solution; add 150-200ml of 25-28% ammonia water dropwise into the silver nitrate solution until the solution is just clear to obtain a silver-ammonia solution.
[0040] 2) Weigh 1.8g analytically pure NaOH and dissolve it in 12L deionized water to make solution A; weigh 18g PVA and dissolve it in 3L deionized water to make solution B; pour the prepared solutions A and B into the reaction kettle respectively , and measure 180mL of analytically pure formaldehyde into the reaction kettle, and stir for several minutes until the entire reduction system is a homogeneous phase.
[0041] 3) Add the silver ammonia solution dropwise to the reducing system, and control the adding rate to 100mL / min. After the silver ammonia solution is added dropwise, it is aged for 45 minutes to obtain a highly dispersed ultrafine silver powder slurry. The temperature of the whole reactio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com