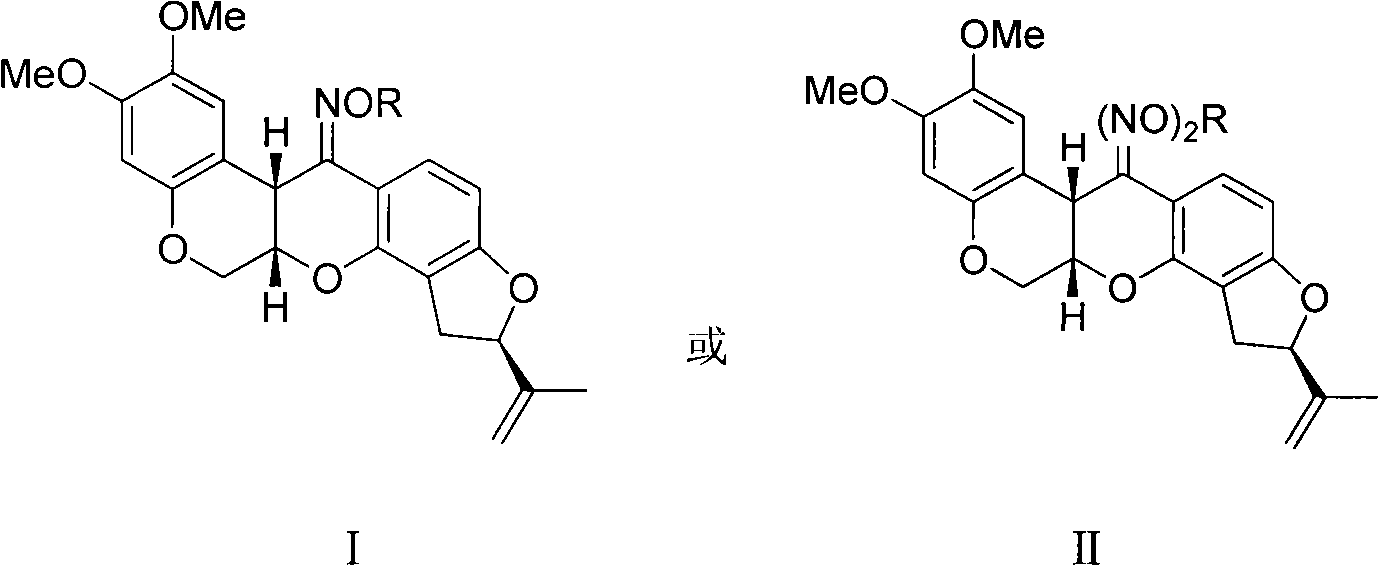
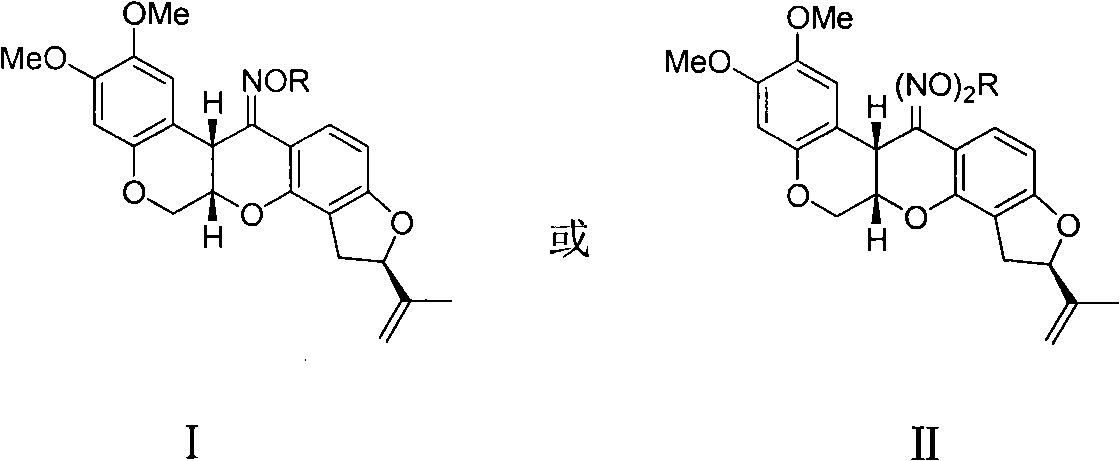
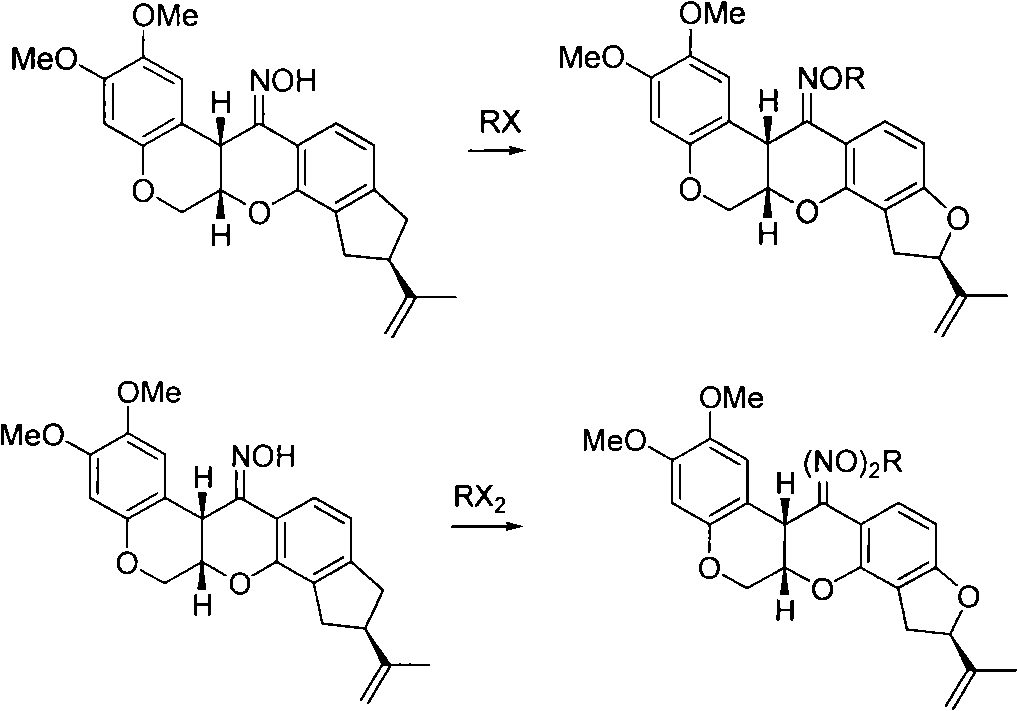
Rotenone oxime ether, preparation method and applications thereof
A technology for rotenone oxime ether and ketoxime ether, which is applied in the field of rotenone oxime ether and its preparation, can solve the problems of oxidative decomposition failure, unstable preparation concentration, and difficulty in standardization, and achieves improved stability, short reaction time and good stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The synthesis of embodiment 1 rotenone oxime methyl ether (2)
[0019]
[0020] 25mL of acetone, 1.00g of rotenone oxime and 0.05g of tetramethylammonium bromide, 2mL of 50% sodium hydroxide solution, stirred for 30min. At 5°C, a solution of 0.38 g of dimethyl sulfate in 5 mL of acetone was added dropwise. After the dropwise addition was completed, the reaction was carried out at 0-5°C, followed by TLC, and the reaction was completed within 10 hours. After filtration, the filtrate was evaporated to dryness under reduced pressure, and the residue was poured into 50 mL of water. Solids were precipitated, filtered, washed with water, and dried to obtain 1.01 g of a light yellow solid with a yield of 98.06%. The melting point is 195-203°C. 1 H NMR (CDCl 3 ), δ: 1.75 (s, 3H, 8'CH 3 ), 2.99(dd, J=8.0Hz, J=16Hz, 1H, 4'-H), 3.28(dd, J=10.0Hz, J=16Hz, 1H, 4'-H), 3.75(s, 3H, OCH 3 ), 3.80 (s, 3H, OCH 3 ), 4.06 (s, 3H, NOCH 3 ), 4.23(d, J=12Hz, 1H, 6-H), 4.49(t, J=2.8Hz...
Embodiment 2
[0021] Example 2 1.5g of ketone oxime, 15ml of DMF, 0.2g of sodium hydroxide, stirred for 20min, 10ml of DMF solution containing 7.2mmol of iodomethane was added dropwise, reacted at room temperature for 3.0h, slowly added saturated saline to the reaction solution, and allowed to stand Separate the layers, filter, dry the filter cake, and recrystallize from ethanol to obtain 2 as a white solid.
Embodiment 3
[0022] Embodiment 3 The synthesis of rotenone oxime propyl ether (3)
[0023]
[0024] 20mL of acetone, 1.00g of rotenone oxime, 0.70g of potassium carbonate, 0.08g of potassium iodide and 0.90g of bromopropane were stirred and reacted under reflux, followed by TLC, and the reaction was completed in 18 hours. The reaction solution was filtered, the filter cake was washed with acetone, the organic phases were combined, evaporated to dryness under reduced pressure to obtain a solid, washed with water, and dried to obtain 1.02 g of a white solid, with a yield of 91.07%. The melting point is 209-214°C. 1 H NMR (CDCl 3 ), δ: 1.01 (t, J=4.4Hz, 3H, -CH 2 CH 3 ), 1.75 (m, 5H, 8'-CH 3 ,-CH 2 CH 3 ), 2.91(dd, J=8.0Hz, J=15.6Hz, 1H, 4'-H), 3.28(dd, J=10.0Hz, J=15.6Hz, 1H, 4'-H), 3.74(s, 3H, OCH 3 ), 3.80 (s, 3H, OCH 3 ), 4.22 (m, 3H, 6-H, OCH 2 ), 4.50(s, 1H, 12a-H), 4.59(dd, J=2.8Hz, J=12.4Hz, 1H, 6-H), 4.78(d, J=2.8Hz, 1H, 6a-H), 4.90(s, 1H, 7'-H), 5.05(s, 1H, 7'-H), 5.14...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com