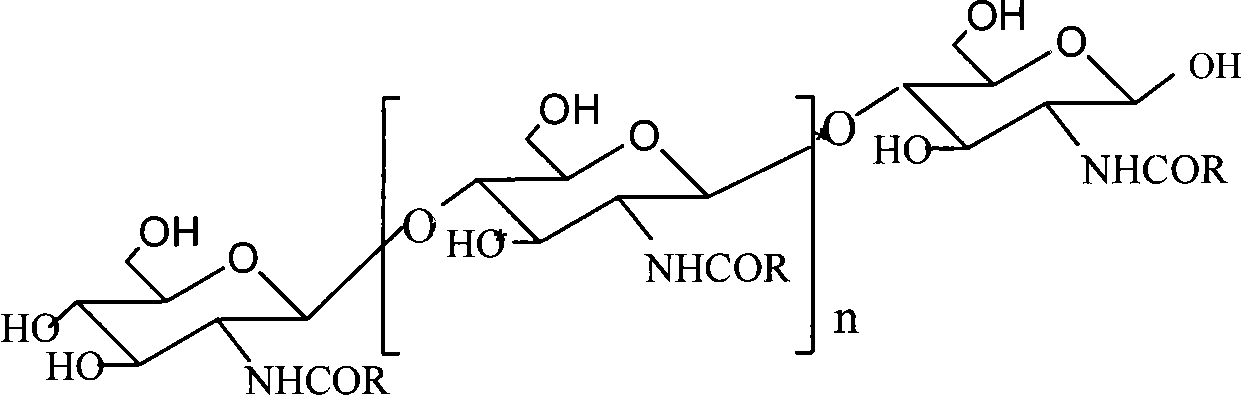
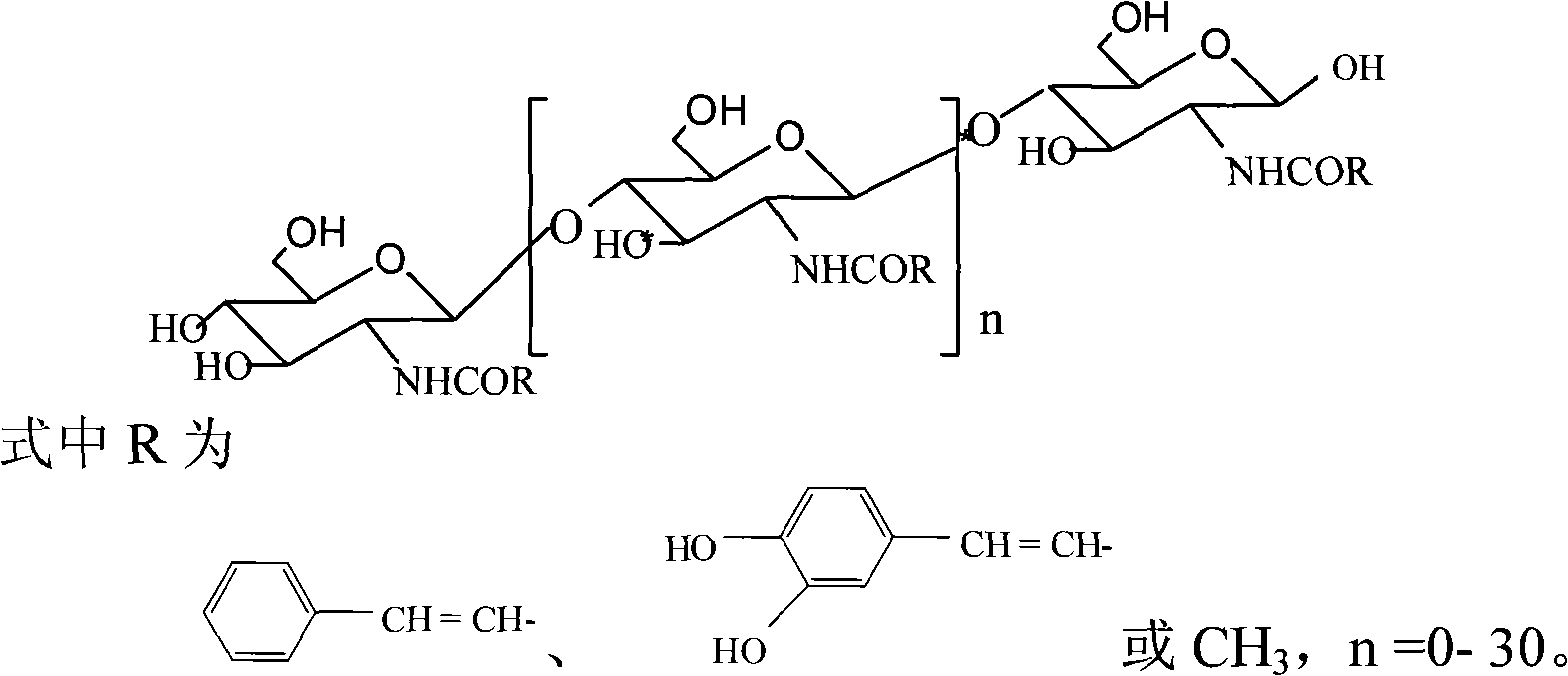
N-phenylpropenoyl chitosan oligosaccharide and preparation thereof
A technology of phenylacryloyl chitosan and phenylacryloyl, which is applied in the field of chitosan and can solve problems such as poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of N-phenylacryloyl chitosan oligosaccharide (ammonolysis method):
[0024] Dissolve 1.48g of phenylacrylic acid in N,N-dimethylformamide (DMF), add 2.7g of p-nitrophenol, stir, add 0.1g of dimethylaminopyridine, dropwise add 2.06g of dicyclohexylcarbodiethylene Dissolve the amine in 5mL of DMF, react at 85°C for 6h after dripping, filter, pour the filtrate into ice water, precipitate crystals, filter with suction, and recrystallize with absolute ethanol to obtain p-nitrophenyl phenylacrylate 2.15 g
[0025] P-nitrophenyl ester can also be prepared as follows:
[0026] Add an appropriate amount of acetone to a dry reaction bottle containing 1.67g of phenylacryloyl chloride under ice bath, stir vigorously, add 10-40mL of anhydrous triethylamine and 2.7g of p-nitrophenol in 20mL of acetone solution dropwise, and react 1- 5h, filtered, the filtrate was poured into ice water, crystals were precipitated, filtered, washed with 5% Na 2 CO 3 Th...
Embodiment 2
[0029] Embodiment 2: 3, the preparation of 4-dihydroxyphenylacryloyl chitosan oligosaccharide (acyl chloride method)
[0030] The preparation of 3,4-dihydroxyphenylacryloyl chloride: 10mL SOCl 2 Put it into the reaction flask equipped with HCl gas absorption device, add 1.67g of 3,4-dihydroxyphenylacrylic acid to it under the protection of nitrogen at -10~5°C, stir, add a few drops of anhydrous DMF, and put it in an oil bath Slowly raise the temperature to 65-85°C, and after refluxing for 3 hours, evaporate the excess SOCl under reduced pressure 2 , to obtain 3,4-dihydroxyphenylacryloyl chloride as a yellow solid, which can be reacted without separation.
[0031] Take 1.98g (10mmol) 3,4-dihydroxyphenylacryloyl chloride and dissolve it in 50mLDMF; another K 2 CO 3Dissolve 2g in 20mL of water, add 1.4g of chitosan oligosaccharide and stir to fully dissolve, slowly add the acid chloride solution to the chitosan oligosaccharide (COS) solution dropwise at low temperature (-10°C ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com